Bacteriophage-mediated transfer of antibiotic resistance genes
Bacteriophages are viruses that infect and can kill bacteria, and are currently considered a promising solution to overcome antibiotic resistance. See how CSIRO researchers are developing the knowledge that could control the spread of these harmful genes from the environment to humans and animals.
The challenge
Bacteria have developed antibiotic resistance over the past decade (early publications can be found here), resulting in adverse health treatment outcomes. Bacteriophages (phages) are viruses that infect and can kill bacteria and are currently considered a promising solution to overcome antibiotic resistance. However, the spread antibiotic resistance genes (ARGs) mediated by phage is also receiving increased attention due to the range of ARGs and virulence factors phage carry. Virulence factors are molecules that help the bacteria infect a host contributing to the spread of ARGs.
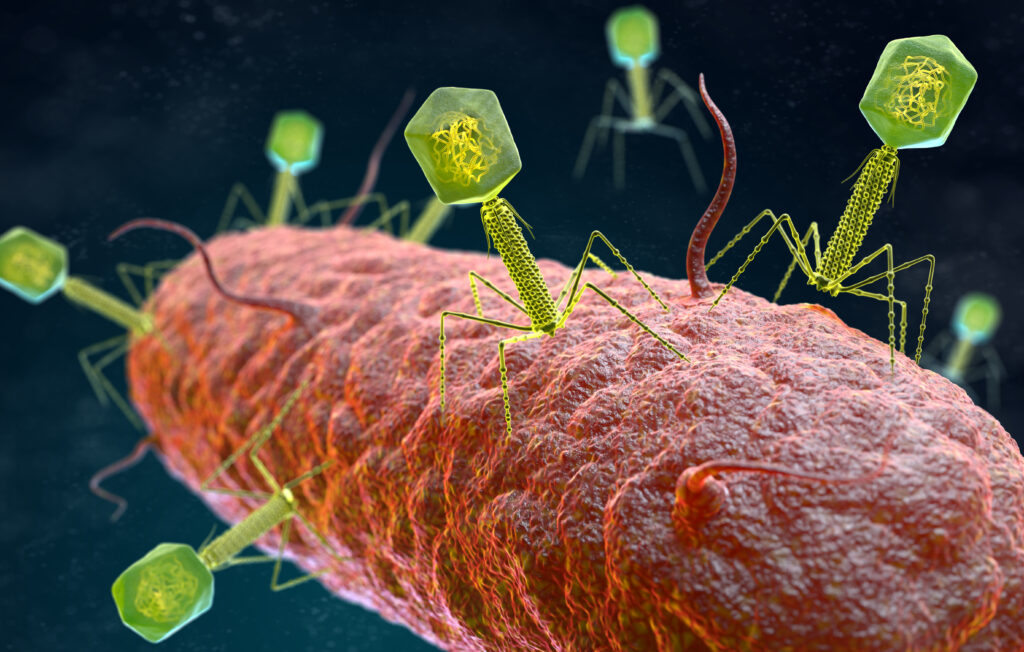
Illustration of the bacteriophage virus (yellow) that infects and replicates within a bacterium (red). Credit – iLexx
Understanding how the spread of ARGs from the environment to humans and animals from organism to organism (horizontal gene transfer) could provide insight to how we can control them. Horizontal gene transfer occurs through three main mechanisms (i.e., conjugation, transduction, and transformation). Transformation (host cell taking up DNA from its surroundings) plays a minor role in ARGs dissemination, whereas bacterial cell to cell gene transfer via conjugation (direct contact) is considered the main route of transfer of ARGs. Transduction (foreign DNA transfer to a host cell by a virus) not only facilitates the spread of ARGs and virulence factors but may also provide host bacteria with adaptive traits such as better survival under antibiotic stress and protection from other phages.
Given the ubiquitous nature of phages and their persistence in the environment, they are increasingly recognised as an environmental reservoir of antibiotic-resistant and virulence genes and spread those genes among bacterial communities. However, very little is known about the overall contribution of phage in the dissemination of antibiotic resistance.
Our response
CSIRO researchers will investigate how phages contribute to the spread of antibiotic resistance and virulence genes. They will use bacteriophages found in aquatic microbiomes to understand the extent and mechanism of gene transfer. This knowledge will provide insight into how to tailor bacteriophage therapies, develop pathogen surveillance and control strategies, and monitoring the spread of these harmful genes from the environment to the gut of humans and animals.
The team
Heng Ku Postdoctoral fellow
Dr Jatinder Sidhu Team leader
Denis Bauer Group leader
