Achievements-RRnD4P Rnd 2
Achievements of Rural R&D for Profit round 2 project
Biocontrol solutions for sustainable management of weed impacts to agricultural profitability (2016-2020)
March 2020
Undertake a literature review on taxonomy and distribution of sowthistle and known natural enemies of the weed in the introduced and native ranges
Taxonomy
Sonchus is a cosmopolitan genus and currently includes 55-60 species (Thompson 2007, 2015a). Most Sonchus species are biennial or perennial with woody roots or rhizomes. Morphologically, species of Sonchus are characterised by their simple, glandular or eglandular hairs, basal and cauline leaves, cymose capitulescence, pedunculated capitula, multiseriate involucral bracts that are reflexed at maturity, yellow flowers, homomorphic achenes that are unbeaked and compressed, pappus of partially persistent almost smooth bristles of two types (Thompson 2007, 2015a). Within Australia, there is one native Sonchus species, S. hydrophilus, and three naturalised species: S. oleraceus, S. asper and S. asper var. asper (Thompson 2007, 2015a).
A recent study has demonstrated that climatic conditions may have driven rapid adaption of S. oleraceus in its introduced ranges in Australia and New Zealand (Ollivier et al. in press). Differences in 20 traits (relating to growth, resource acquisition, reproduction, phenology and defence) amongst 14 populations of the herbaceous plant S. oleraceus L. (Asteraceae) across its native (Europe and North Africa) and introduced (Australia and New Zealand) ranges were investigated in a glasshouse experiment. Introduced S. oleraceus plants possessed higher leaf and stem dry matter content, greater number of leaves and were taller at first flowering stage than plants from the native range.
Sonchus oleraceus belongs to the subtribe Hyoseridinae (synonym Sonchinae) in the tribe Cichorieae, subfamily Cichorioideae in the family Asteracea (Killian et al. 2009). The genus Launaea is closely related to Sonchus. Launaea sarmentosa is the only species of this genus that is native to Australia (Thompson 2015b). Reichardia is the next genus related to Sonchus and Launaea. It is a small genus of approximately eight species that is native to the Mediterranean and two species of this genus, R. tingitana and R. picroides have naturalised in Australia (Thompson 2015c). Hyoseris and Aposeris are the last two genera in the subtribe Hyoseridinae. Based on recent taxonomic revisions (Kilian et al. 2009; Thompson 2015d), there are no species of Hyoseris which currently occur in Australia. There is no record of Aposeris foetida, the sole species in this genus, occurring in Australia.
Distribution
Sonchus oleraceus has a very broad global geographic distribution and can be found in temperate, tropical and subtropical climates (Kilian et al. 2009) (Fig. 1). It is native to Europe, Macaronesia (Madeira Islands, Canary Islands), North Africa (Algeria, Egypt, Libya, Morocco, and Tunisia) and South-Western Asia (CABI 2020). The greatest diversity of Sonchus is regarded to be in the Western Mediterranean (Mejias & Andres 2004).
In Australia, S. oleraceus is widespread and found in all States and Territories but appears to be most prevalent in the southern half of the continent. There are more than 33,000 records of S. oleraceus in the Atlas of Living Australia (ALA 2017) (Fig. 2).
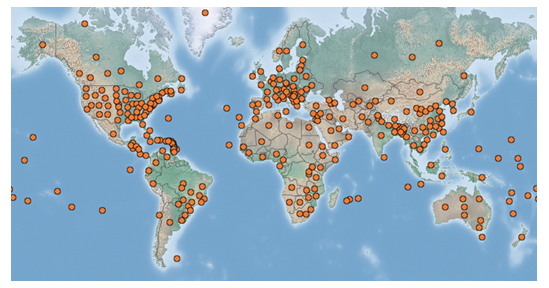
Figure 1. Graphical representation of the worldwide distribution of Sonchus oleraceus (common sowthistle) (CABI 2020).
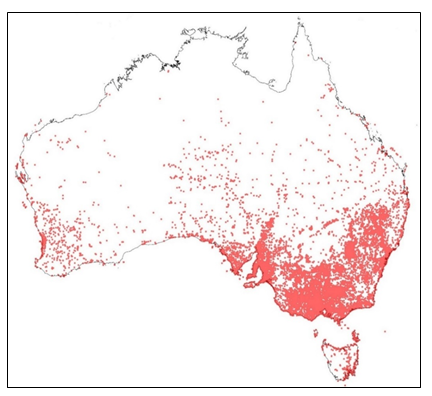
Figure 2. Occurrence records of Sonchus oleraceus (common sowthistle) in Australia (ALA 2020).
Natural enemies
The comprehensive review of the literature we undertook revealed that 23 fungi and 7 insects are recorded on S. oleraceus in Australia. In Europe, 22 fungi and 75 insects, different to those reported in Australia, have been recorded as infecting and feeding on S. oleraceus, respectively. Among these fungi found on S. oleraceus in the native range, Bremia lactucae (species specific strains if they exist), Bremia sonchi, Septoria sonchifolia and Entyloma bullulum were singled out as those with the most promise as potential biocontrol agents. The specialist insects Tephritis dilacerata, Contarinia schlechtendaliana, Cystiphora sonchi, Botanophila sonchi and Aceria sonchi, were also of interest because of their potential restricted host range.
More recently, a PhD study affiliated with this project combined literature and field surveys, to document 17 phytophagous arthropod species, mostly generalists of exotic origin, able to feed and develop on S. oleraceus in Australia (Ollivier et al. in preparation). The capitula/flower heads were the most damaged plant part while stems were relatively free from insects, except aphids.
Define goals for management of sowthistle
Since both S. oleraceus and C. bonariensis are weeds affecting cropping systems, a combined survey of key stakeholders in the grains industry on the impacts and desired management goals for these weeds, was performed. See Define goals for management of fleabane.
Nominate sowthistle as a biocontrol target
The project prepared the documentation to support the nomination of S. oleraceus as a target for biocontrol. The documentation was submitted to the IPAC (now EIC), by the New South Wales Department of Primary Industries in August 2017 and endorsed by the Committee in November 2017 (Hunter and Ireland 2017).
Conduct genetic analysis on samples of sowthistle from different regions in Australia and the native range
We have analysed a large genomic dataset to determine the origins of S. oleraceus introductions into Australia in order to guide field surveys for natural enemies with potential for biocontrol. Detailed results from this study are in Encinas-Viso et al. (in preparation).
Leaf material of S. oleraceus was collected from 27 sites (6-18 plants per site) across its native range in Europe and North Africa as well as 17 sites (9-15 plants per site) across Australia (Fig. 3). Each leaf was subsampled and forwarded to Diversity Arrays Technology (DArT) in Canberra for DNA extraction, quantification and genotyping. The Single Nuceotide Polymorphism (SNP) dataset from DArT consisted of 33,959 loci. The SNP dataset was filtered using a call rate threshold > 95%, leaving us with 11,405 loci. We removed monomorphic loci (as a result of removal of individuals), outliers based on individual’s observed heterozygosity to eliminate potential mixed genomes and duplicate individuals, leaving 2883 SNP markers and 547 individuals.
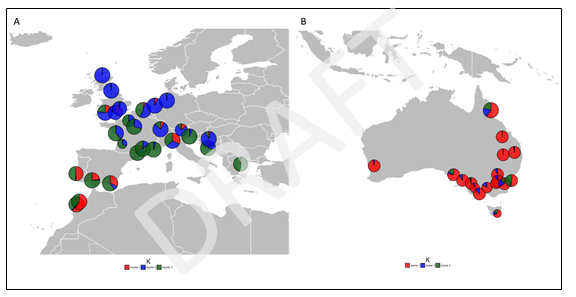
Figure 3. Geographic map of sampling locations of Sonchus oleraceus in A) native range (Europe/North Africa) and B) introduced range (Australia). Pie charts show STRUCTURE results for K=3 considering all sampling locations (Fig. 4). Circle sizes represent relative number of samples per population.
Our STRUCTURE analysis found low levels of genetic structure within the native and introduced range of S. oleraceus indicating a clustering level of (Figs 3, 4), according to method. The analysis clearly separated the native (Europe/North Africa) and introduced (Australia) ranges of S. oleraceus. There were some exceptions to this, showing mixed assignments in Southern Europe and North Africa (Spain, Portugal and Morocco) as well as two populations in the introduced range (ACT and NSW). The analysis also showed that for (second highest value) the native range separates into two genetic clusters: Southern Europe and Northern Europe, revealing subpopulation structure in Europe (Fig. 4c).
The PCA analysis confirmed the patterns found by STRUCTURE showing a separation of the large genetic clusters in Europe/North Africa as well as the genetic cluster in Australia (Fig. 4b). The PCA explained 41.49% of the total genetic variance for the two first principal components (PC) showing genetic variability among the native and introduced ranges. The first PC differentiates Europe/North Africa and Australia, while the second PC separates the native range into Northern and Southern Europe (the latter including North Africa samples) showing partial overlap with Admixed zone sampling locations.
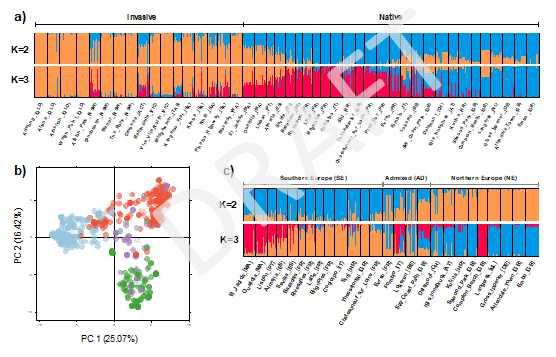
Figure 4. STRUCTURE output and PCA results for 2,883 unlinked loci for sampled populations of Sonchus oleraceus across the native (Europe/North Africa) and introduced (Australia) ranges. a) STRUCTURE output for K=2 (optimal clustering level) and K=3 considering all sampling locations. b) Principal component analysis considering all sampling locations; Northern Europe (green), Southern Europe (red), Admixed zone (purple) and Australia (light blue), based on STRUCTURE output considering only the native range for K=2 (optimal clustering level). c) STRUCTURE output for K=2 (optimal clustering level) and K=3 considering only sampling locations of the native range (Europe/North Africa).
To test multiple invasion scenarios of S. oleraceus into Australia we used an approximate Bayesian computation framework (ABC). Evolutionary relationships among the sampled populations were also evaluated with the software TreeMix. We simulated invasion scenarios from non-admixed and admixed source populations of their native range including multiple introductions to Australia from Europe (including North African populations). We selected the most likely invasion scenario using the abcrf R package, which uses a novel approach based on random forest (RF) machine learning algorithms. For detail on methods and results see Encinas-Viso et al. (in preparation). The ABCRF analysis shows that the most likely invasion scenario of S. oleraceus into Australia was an initial introduction from the Northern Europe cluster and a secondary, more recent introduction from Southern Europe/North Africa cluster. The TreeMix and STRUCTURE analyses also clearly supported the scenario of multiple introductions from different regions of the native range and post-introduction admixture.
Undertake bioclimatic models to identify optimal locations and conduct native range surveys and host-specificity tests for potential biocontrol agent(s) and import at least one potential agent in quarantine
Bioclimatic modelling
The approaches taken for the bioclimatic modelling of S. oleraceus were like those used for Lycium ferocissium: Match Climates and Compare Locations models were developed for S. oleraceus using the CLIMEX package (Kriticos et al. in preparation). The growth rate of S. oleraceus at different temperatures was also measured in an experiment (Figs 5-7).
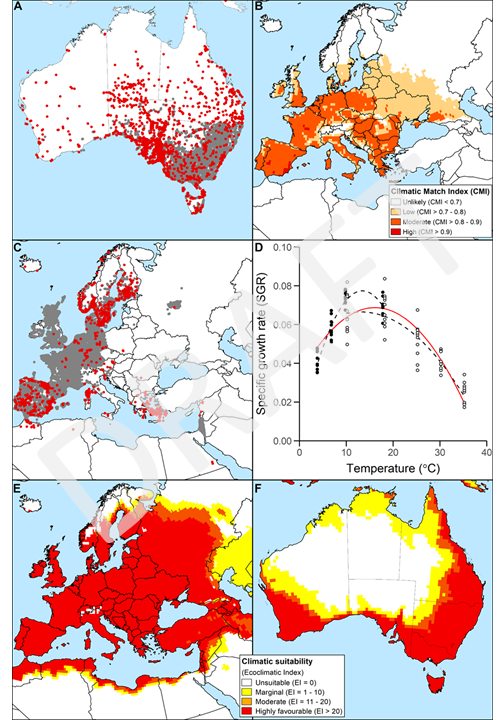
Figure 5. Sonchus oleraceus‘ current distribution in (A) Australia and (C) the native range of Europe. Preserved and living specimen records (red points) projected atop observational records (grey points) (GBIF.org 24th July 2018). (B) CLIMEX Match Climates model, as projected for Europe. (D) Temperature response curve. Final fitted polynomial model incorporating both datasets shown as a red line. Projected climatic suitability from the CLIMEX Compare Locations model projected for Europe (E) and Australia (F).
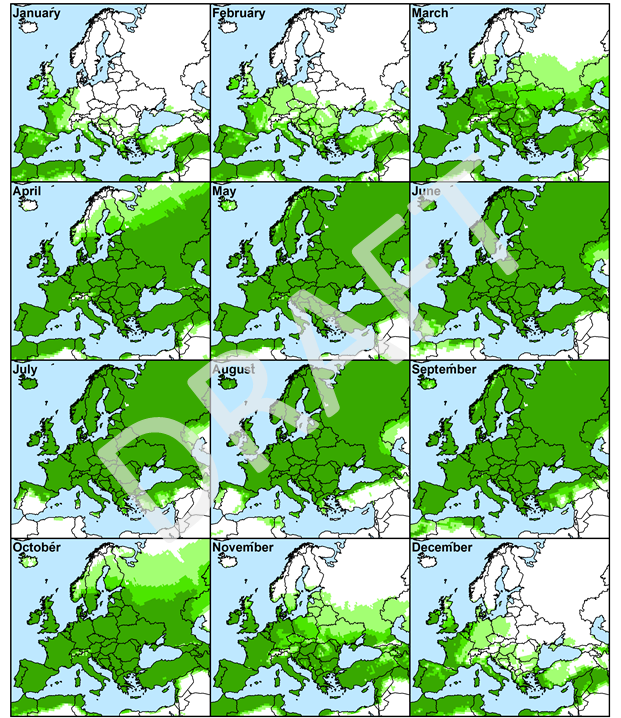
Figure 6. Monthly Growth Index values in native range for guiding when and where to survey for natural enemies on Sonchus oleraceus. Values are averaged across five years from 2012 to 2017. Surveying is recommended within areas in which the Ecoclimatic Index is most suitable, indicating potential for year-round survival. Increased intensity of green colour indicates higher climatic suitability.
Figure 6. Monthly Growth Index values in native range for guiding when and where to survey for natural enemies on Sonchus oleraceus. Values are averaged across five years from 2012 to 2017. Surveying is recommended within areas in which the Ecoclimatic Index is most suitable, indicating potential for year-round survival. Increased intensity of green colour indicates higher climatic suitability.
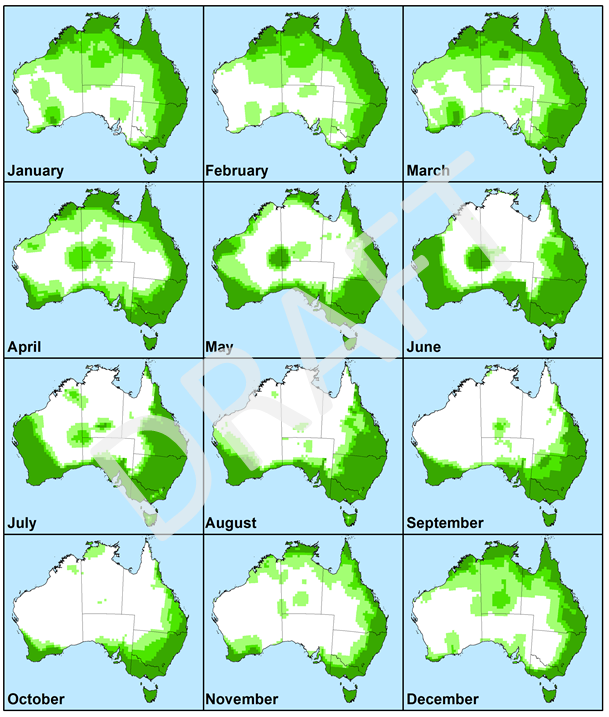
Figure 7. Monthly Growth Index values in Australia for guiding when and where to release biocontrol agents on Sonchus oleraceus. Values are averaged across five years from 2012 to 2017. Agents would only be deployed in areas in which the Ecoclimatic Index was most positive, indicating potential for year-round survival. Increased intensity of green colour indicates higher climatic suitability.
Native range surveys
Native range surveys were conducted between March 2017 to March 2020, mainly in spring and autumn. The selection of areas surveyed was mostly based on parallel bioclimatic modelling that identified northern Africa and the Southern European edge of the S. oleraceus range as most climatically similar to regions where the species is a problem in Australia (Kriticos et al. in preparation). A genetic analysis also identified this broad region as one of the likely sources of S. oleraceus populations in Australia (Encinas-Viso et al. in preparation). Surveys were thus concentrated in southern Portugal, southern France, northern Italy, the Balkans and Greece, with a particular attention in Morocco and southern Spain (Lesieur et al. in preparation). Surveys were also performed in the Canary Islands, in the Macaronesian region off the northern African west coast. Short surveys were also conducted in northern France, Belgium, the Netherlands and Germany. Some sites were visited more than once across the years, especially in Southern Spain, Morocco and within the vicinity of Montpellier, southern France. In the latter, surveys were conducted regularly during the year to gather phenology data on the natural enemies present.
Pathogens
Of the many fungi recovered from disease symptoms during the surveys, 10 were classified as pathogenic once their identification was confirmed. Examination of morphological characters, supplemented with sequencing, were performed to confirm their identity. The rust fungus, Miyagia pseudosphaeria, which already occurs in Australia, was found in several of the regions surveyed. Another rust fungus, Coleosporium sonchi, was found at one site in the Netherlands and four sites in Brittany (France). Prior to sequencing, the fungus could not be morphologically identified because teliospores were not present. A downy mildew morphologically identified as Bremia sp. was found at several sites and is in the process of being sequenced for species identification. A powdery mildew, Golovinomyces sonchicola or G. cichoracearum, and the leaf spot fungus Alternaria sonchi, were found to be common. Sequences of another leaf spot fungus, originally identified as Ascochyta sp. using morphological characters, were found to be highly similar to that of Didymella rosea. Two leaf spot fungi, morphologically identified as generalist Phoma species, were molecularly identified as Didymella glomerata (or Didymella fabae or Phoma sp.) and Didymella sp. Other leaf spot fungi isolated were Ramularia helminthiae and Septoria sonchi.
Insects
Fifty-eight phytophagous insect species, across seven orders, but primarily Diptera and Lepidoptera, and one mite species were collected on S. oleraceus during the surveys (Lesieur et al. in preparation). Identifications were obtained by a combination of morphological and molecular approaches. Most of the species collected were polyphagous or oligophagous species (n = 38 (65%) and n = 2 (3%), respectively). Only a few insects specialized on the genus Sonchus were found (n = 2 (3%)). For three species, the host range was unknown due to the lack of identification to species level or the lack of information within the literature. Many of the species were ectophagous sucking and chewing insects (31% and 25%, respectively), while the endophagous guilds was dominated by mining insects. The most damaged part of the plant was the flower heads, with 39 % of the recorded species possibly using it as feeding resource. No severe damage was observed on roots and only few species appeared to be associated with this feeding niche. Four insect species offered the most promise as potential biocontrol agents based on their identities, data from the literature and the damage on S. oleraceus observed in the field. The fruit fly Tephritis formosa (Diptera: Tephritidae) was commonly found and infested capitula of S. oleraceus and S. asper during the survey. Another fruit fly, Campiglossa producta was only collected in the Canary Islands (Spain). The syrphid fly Cheilosia latifrons (Diptera: Syrphidae), for which larvae mined the stems and the root-crown, was commonly found around Montpellier. The species was also collected in northern Spain. The leaf gall midge, Cystiphora sonchi (Diptera: Cecidomyiidae) was widespread across the sites surveyed.

Figure 8. Cheilosia latifrons. A. Larva mining the stem of a flowering plant; B. Larva mining the crown of a rosette; C. adult (female).
Host-specificity tests for potential biocontrol agents
The proposed list of non-target species for host-specificity testing of candidate biocontrol agents for S. oleraceus was submitted to DAWE in December 2018 for posting on their website for feedback (Hunter and Morin 2018). Initial host-specificity testing with fungal pathogens and insects with potential for biocontrol in Australia was performed at the CSIRO European Laboratory in Montpellier, France.
Pathogens
Six pathogenic fungi recovered from S. oleraceus were tested: Alternaria sonchi, Bremia sp., Coleosporium sonchi, Didymella rosea, Ramularia helminthiae and Septoria sonchi (Lesieur et al. in preparation). These tests included S. oleraceus as a positive control and two closely related species that are native in Australia; Sonchus hydrophilus and Actites megalocarpus. All fungi infected and caused disease symptoms on all three plant species, thus showing no promise for the biocontrol of S. oleraceus in Australia (Fig. 9).
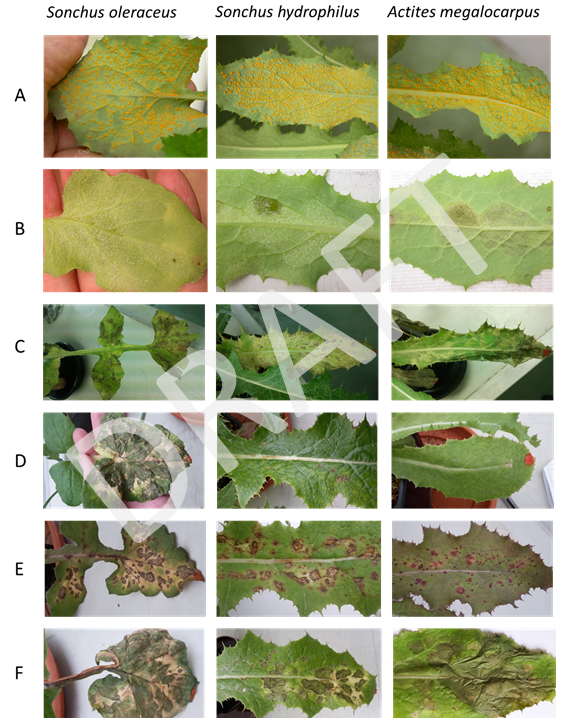
Figure 9. Disease symptoms observed during initial host-specificity tests on Sonchus oleraceus and closely related species inoculated with the following fungi: A. Coleosporium sonchi, B. Bremia sp., C. Septoria sonchi, D. Didymella rosea, E. Alternaria sonchi, F. Ramularia helminthiae.
Insects
An abridged phylogenetically based test list comprising ten plant species was used for initial screening of the specificity on the first insect candidate agent found, the gall midge, C. sonchi (Fig. 10). Results of no-choice and choice tests showed that the midge could develop species in the same sub-tribe as S. oleraceus, including the two native species S. hydrophilus and A. megalocarpus (Lesieur et al. in review). Based on results from these tests, a decision was made to concentrate initial testing of the other promising candidate insects on these two important native species.
The three other insect species tested, T. formosa, C. producta and C. latifrons were found to oviposit, develop and complete their life cycle on S. oleraceus and the two native species.
In addition to the above, host specificity tests focused on candidate pathogen and insect agents, novel methodologies were developed to understand how multiple trophic levels may interact in the native vs invaded range to influence the efficacy of weed biocontrol (Ollivier et al. 2020).

Figure 10. Galls produced by the leaf gall midge, Cystiphora sonchi on non-target native species during preliminary host-specificity testing; a. Sonchus hydrophilus; b. Actites megalocarpus.
Pending risks to non-target plants are acceptable, submit application to the Commonwealth regulators seeking approval to release at least one potential agent. Upon receiving approval, rear and release biocontrol agent(s)
None of the fungal pathogens and insects found on S. oleraceus in the native range, for which initial host-specificity testing was performed, are specific enough to be pursued further as possible biocontrol agents in Australia. While there may be additional natural enemies that could be found to have potential for biocontrol, this scenario is unlikely considering the considerable survey efforts undertaken in this project.
Discussions with GRDC (the principal co-investor for work on S. oleraceus) have commenced to explore meaningful ways forward for research on biocontrol of other grains weeds in the new Rural R&D for Profit project (Agrifutures Australia Project number: PRJ-12377).
Explore options for integration of biocontrol with other management techniques
Options for integration of biocontrol with other management techniques were jointly explored for Conyza spp. and S. oleraceus since they have similar impacts grain production systems. See Fleabane – Explore options for integration of biocontrol with other management techniques.
References
ALA (2020) Sonchus oleraceus: Common sow thistle. Atlas of Living Australia website at https://bie.ala.org.au/species/https://id.biodiversity.org.au/node/apni/2895772 Accessed 15 March 2020.
CABI (2020) Datasheet: Sonchus oleraceus (common sowthistle). In: Invasive Species Compendium. http://www.cabi.org/isc/datasheet/50584
GBIF.org (24th July 2018) GBIF Occurrence Download https://doi.org/10.15468/dl.pbd67p.
*Hunter GC, Ireland KB (2017) Nomination of a target weed for biological control: Sonchus oleraceus L. (Asteraceae). Prepared by CSIRO.
*Hunter GC and Morin L (2018) Proposed plant host test list for assessing risk of candidate biological control agents for Sonchus oleraceus. Prepared by CSIRO.
Kilian N, Gemeinholzer B, Lack HW (2009) Cichorieae. In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds) Systematics, Evolution and Biogeography of Compositae. International Association for Plant Taxonomy, Vienna, pp 343–383.
*Lesieur V, Thomann T, Ollivier M, Raghu S (in review) Making host specificity testing more efficient: exploring the use of abridged test plant lists.
Mejias JA, Andres C (2004) Karyological studies in Iberian Sonchus (Asteraceae : Lactuceae): S. oleraceus, S. microcephalus and S. asper and a general discussion. Folia Geobotanica, 39, 275–291.
*Ollivier M, Kazakou E, Corbin M, Sartori K, Gooden B, Lesieur V, Thomann T, Martin J-F, Tixier M-S (in press) Trait differentiation between native and introduced populations of the invasive plant Sonchus oleraceus L. (Asteraceae). Neobiota
*Ollivier M, Lesieur, V., Raghu, S and Martin, J-F (2020). Characterizing ecological interaction networks to support risk assessment in classical biological control of weeds. Current Opinion in Insect Science 38: 40–47.
Thompson IR (2007) A taxonomic treatment of tribe Lactuceae (Asteraceae) in Australia. Muelleria, 25: 59–100.
Thompson IR (2015a) Sonchus. Flora of Australia 37: 117–121.
Thompson IR (2015b) Launaea. Flora of Australia 37: 123.
Thompson IR (2015c) Reichardia. Flora of Australia 37: 124–125.
(asterisk indicates outputs from this project)
