Previous research (2017-2024)
Trust-funded sub-project
The foliar blight fungus Venturia paralias (previously referred to as Passalora euphorbiae) was selected as the most promising candidate agent of sea spurge to pursue for the sub-project supported by the NSW Environmental Trust because it:
- has been shown to be highly host-specific in preliminary tests, although more tests have to be performed, especially on Australian native Euphorbia , to gather all information required to support an application for release in Australia,
- damages leaves and stems, which are present all-year round,
- has been observed to cause severe infections on sea spurge in the field in the native range,
- should, if approved for release in Australia, cause disease symptoms on sea spurge over most of the year, due to the high humidity present in coastal areas where the weed occurs.
In November 2020, Venturia paralias was approved for release in Australia.
Phase 1 (July 2017 – June 2019)
The key activities of this phase of the sub-project were to:
- Undertake comprehensive host-specificity testing of the candidate biocontrol agent on closely-related plant species to sea spurge, including selected Australian native species.
- Prepare and submit to the relevant authorities an application for its release in Australia for the biocontrol of sea spurge, pending results indicate that the fungus does not pose a threat to non-target species.
Phase 2 (January – September 2021)
The key activities of this phase of the sub-project were to:
- Develop an efficient method for mass production of the agent.
- Identify sea spurge infested sites in NSW that are not under active control to test different release methods for the agent.
- Evaluate efficacy of the release methods by assessing establishment of the agent in the field.
Phase 3 (July 2021 – June 2024)
In this phase of the sub-project, releases of the approved biocontrol agent occured in sea spurge infestations in regions of Tasmania and Victoria that are the source of recurrent infestations in NSW due to seed dispersal on ocean currents. Various agencies and groups, such as Coastcare, Landcare Australia, Landcare Tasmania, Parks Victoria, Tasmanian Parks and Wildlife Services, Cradle Coast Authority, Great Ocean Road Coast and Parks Authority, and the Sea Spurge Remote Area Team (SPRATS) volunteer group, in these states have committed to provide in-kind support to the sub-project.
The key activities of this phase of the sub-project were to:
- Mass-produce the agent.
- Gather baseline data on sea spurge population at monitoring sites in Tasmania and Victoria and release the agent.
- Release the agent at other sites, with involvement of local communities wherever appropriate.
- Evaluate establishment, spread and initial impact of the agent at monitoring sites in NSW, Tasmania and Victoria.
Phase 1: Summary of achievements
October 2021
Cultures of the fungus Venturia paralias (previously referred to as Passalora euphorbiae) stored since 2009 at the CSIRO European Laboratory in France, were imported in the CSIRO quarantine in Canberra in October 2017. A robust and reliable methodology for host-specificity testing has been developed by optimising the culturing, inoculation and assessment protocols. Propagating material for 40 non-target plant species of the test list (76 different accessions) were sourced and successfully propagated. The test list comprised species across the families Euphorbiaceae, Picrodendraceae and Phyllanthaceae.

Results of host-specificity testing indicated that the fungus can only severely damage sea spurge. Visible necrotic lesions developed 11-12 days after inoculation of sea spurge and prolonged disease progression allowed the fungus to move from infected leaves to stems and girdle them, leading to eventual stem collapse and toppling of plants. The fungus only caused leaf lesions, albeit restricted, on one non-target species, Euphorbia segetalis, the most phylogenetically related species to sea spurge. This species is naturalised in Australia and recorded as an environmental weed, although it is very uncommon. The fungus was also capable of penetrating leaves and developing a few subcuticular amoeboid primary hyphae on Euphorbia myrsinites, Euphorbia helioscopia and Mercurialis annua, but these did not develop any further. On all other non-target species tested, conidia germinated on leaf surfaces and germ tubes formed appressoria, but the fungus was never observed to penetrate or proliferate inside leaves.
An application to release this fungus in Australia was submitted in September 2019 to the Federal Department of Agriculture, Water and the Environment (DAWE). Permission to release in Australia obtained in November 2020 at https://www.agriculture.gov.au/biosecurity/risk-analysis/biological-control-agents/risk-analyses/completed-risk-analyses/ra-release-venturia-paralias
Publications:
Crous PW et al. (2020) Fungal Planet description sheets: 1042–1111. [Hunter GC, Zeil-Rolfe I, Jourdan M, Morin L. Venturia paralias-sheet 1109]. Persoonia 44:301-459. https://www.ingentaconnect.com/content/nhn/pimj/pre-prints/content-nbc-persoonia-0550
Hunter GC, Zeil-Rolfe I, Jourdan M, Morin L (2021) Exploring the host range and infection process of Venturia paralias isolated from Euphorbia paralias in France. European Journal of Plant Pathology 159: 811-823.
Phase 2: Summary of achievements
October 2021
Three different approaches to produce the sea spurge biocontrol agent, the fungus Venturia paralias, for field releases were tested. The most efficient method was to produce sporulating cultures of the agent on agar medium in plates and drying the cultures at room temperature for 24-48 hrs and storing at 4°C until ready for use.
Following consultation with a range of land managers (councils, national park), three sites on the NSW South Coast with an adequate number of sea spurge plants were identified to test release methods: Cudmirrah Beach, Cunjurong Beach and Brou Beach.
Up to six different methods for releasing the agent were tested across the three sites. The release methods that involved spraying spore suspensions made from fresh and dried cultures of the agent resulted in more stem lesions developing than any of the other methods tested.

Phase 3: Summary of achievements
June 2025
Biocontrol agent releases undertaken by community members
Over the three-year sub-project focused on the biological control of sea spurge, the project team successfully cultured, maintained, and mass-produced the biocontrol agent Venturia paralias for release into the Australian environment. Using the methodology developed during this sub-project, V. paralias was cultured on half-strength Potato Dextrose Agar (½ PDA; Sigma-Aldrich, St. Louis) and incubated at 20 °C for 3–4 weeks to generate sufficient fungal biomass. After incubation, fungal spread plates were dried in either a Laminar Flow Cabinet or Biological Safety Cabinet for 48 hours. The dried, sporulating cultures were then scraped from the plate surfaces and transferred to 50 mL centrifuge tubes, typically using material from 2–3 plates per tube.

Figure 1. Cultures of Venturia paralias (A) grown in a UV cabinet (B). Cultures were regularly checked for mites and secondary infection (C). When ready, cultures were dried in a laminar flow cabinet (D).
Between December 2021 and June 2024, a total of 543 vials (50 mL each) containing dried V. paralias fungal material were distributed to registered community participants—325 to Tasmania and 218 to Victoria.
Biocontrol release kits were provided to eight types of organisations and participants, including Aboriginal Land Councils, community groups, federal and state government agencies, Landcare, local government, private landowners, and public land managers. Seven of these organisation types were represented in Tasmania, and six in Victoria.
Each participant received hard copies of the release instructions and access to an online video demonstrating the preparation and field inoculation process: https://youtu.be/Mv8CCnjIlV8.
Field releases of the biocontrol agent began in December 2021 and continued through to May 2024. In Victoria, releases occurred at 128 sites:
- 3 sites in 2021
- 68 sites in 2022
- 23 sites in 2023
- 34 sites in 2024
In Tasmania, releases were conducted at 103 sites across the west, north, and east coasts, including King Island, Flinders Island, and Cape Barren Island:
- 9 sites in 2021
- 55 sites in 2022
- 19 sites in 2023
- 20 sites in 2024
In total, 231 release sites were established across both states during the sub-project.
Community participants returned to 128 sites to monitor disease symptoms caused by the biocontrol agent, typically by photographing inoculated sea spurge plants several weeks post-inoculation. Unfortunately, 10 sites had been washed away by the ocean. CSIRO project staff reviewed monitoring data from the remaining 118 sites and confirmed successful infection and establishment of V. paralias at 72 sites (61%) (see Fig. 2).
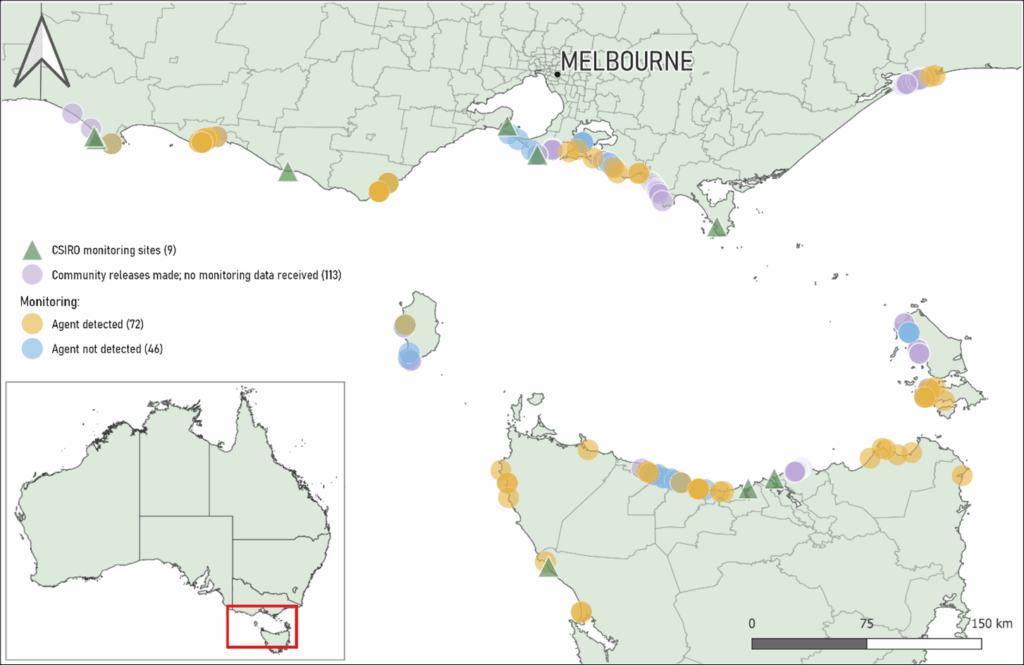
Figure 2. Map of Victoria and Tasmania indicating the locations of nine CSIRO monitoring sites (green triangle), field releases of the agent undertaken by registered community participants (purple circle), field releases of the agent undertaken by registered community participants who provided subsequent monitoring feedback and where the agent was detected (orange circle), and field releases of the agent by registered community participants who provided monitoring feedback but where the agent was not detected (blue circle). Scale = 150 km.
Assessment of biocontrol agent establishment at the fixed monitoring sites
Victoria:
Between February and March 2022, six dedicated monitoring sites were established across Victoria: Whites Beach, Shelley Beach, London Bridge (Port Campbell National Park), Sand Island (Unreserved Crown land managed by Parks Victoria), Bushrangers Bay, and Oberon Bay (Wilsons Promontory National Park) (Fig. 2, 3, 4). Monitoring was conducted once or twice annually (Autumn and Spring), resulting in four post-release assessments over the three-year project.
Each site included three 10 m transects, with five 1 m² monitoring plots per transect—totalling 15 plots per site and 90 plots across all six sites. The biocontrol agent V. paralias was spray-inoculated onto sea spurge plants in each plot during site establishment in 2022.
By September 2022 (6–7 months post-release), disease symptoms were evident at all sites. Of the 90 plots:
- 39% (35 plots) showed stem lesions,
- 36% (32 plots) showed leaf lesions,
- 57% (52 plots) showed either leaf and/or stem lesions.
Subsequent assessments confirmed continued establishment of the agent:
- May 2023 (14–15 months post-release): Agent detected in 73% (55/75) of plots (London Bridge excluded due to unsafe conditions).
- October 2023: Agent detected in 64% (48/75) of plots (Whites Beach excluded due to site loss).
- April 2024 (24–26 months post-release): Agent detected in 71% (53/75) of plots.
These results provide strong evidence that V. paralias has successfully established in Victoria.
Additionally, the agent has spread beyond the initial monitoring plots. By October 2023, it had dispersed (Fig. 4):
- 200–250 m from Bushrangers Bay, Whites Beach, and Sand Island,
- Up to 1,000 m from Oberon Bay,
- Up to 1,500 m from Shelley Beach.

Figure 3. Victorian sea spurge monitoring sites and infected sea spurge symptoms. (A) monitoring site establishment and baseline assessment at Sand Island (February 2022), (B) stem lesion (arrow) on sea spurge at Sand Island (September 2022), and (C) leaf lesions (arrow) caused by V. paralias on sea spurge at Sand Island (October 2023), and (D) final assessments at Bushrangers Bay (April 2024).
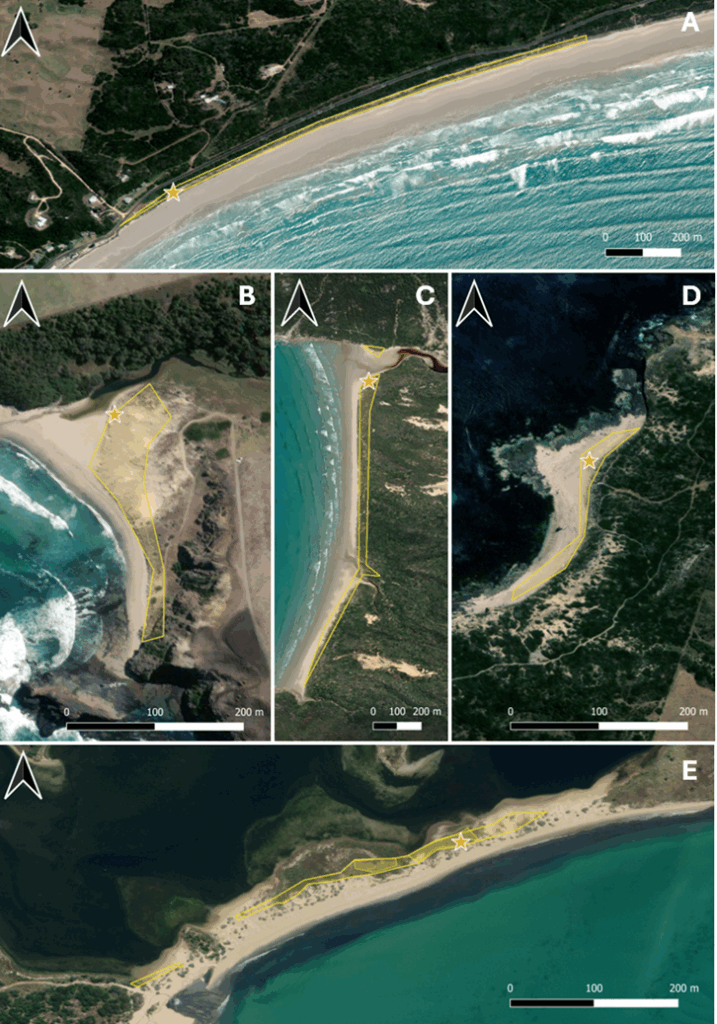
Figure 4. Natural spread of Venturia paralias from monitoring sites in Victoria. (A) Shelly Beach, (B) Bushrangers Bay, (C) Oberon Bay, (D) Whites Beach, and (E) Sand Island. Venturia paralias spread was not assessed at London Bridge. Stars indicate the original CSIRO release and monitoring site, diagonal-shaded areas indicate medium-low levels of Venturia paralias infection (A-E), cross-shaded areas indicate high levels of infection (C, E).
Tasmania:
Three monitoring sites were established in Tasmania for this sub-project: Bakers Beach (Narawntapu National Park) and Low Head (Low Head Conservation Area) on the north coast, and Duck Creek on the west coast. These sites were set up in late October 2021, approximately four to five months prior to the Victorian monitoring sites, allowing for an additional assessment—bringing the total to five monitoring events in Tasmania.
The first assessment, conducted in March 2022 (five months post-release), revealed a high incidence of stem lesions (84%, 38/45 plots) but no leaf lesions across the 1 m² monitoring plots. Leaf lesions were first observed during the second assessment in November 2022, appearing in 96% (43/45) of plots, while stem lesions were present in 44% (20/45) (Fig. 5C, 4D). From this point forward, the pathogen was consistently detected across 90–93% of plots in all subsequent assessments, confirming strong establishment at all three sites.
The biocontrol agent also began to disperse beyond the monitoring plots. During the November 2022 assessment, infected sea spurge plants were identified up to 100 m from Bakers Beach and 200 m from Low Head (Fig. 6). Continued observations throughout the project confirmed ongoing spread, indicating promising potential for further dispersal of V. paralias along the Tasmanian coastline.

Figure 5. Tasmanian sea spurge monitoring sites and infected sea spurge symptoms. (A) monitoring site establishment at Low Head (October 2021), (B) typical stem lesion (arrow) caused by Venturi paralias on sea spurge at Low Head (March / April 2022), Typical leaf lesions (arrow) caused by V. paralias on sea spurge (C) within the monitoring site at Bakers Beach (November 2022), and (D) outside of the monitoring site at Low Head (November 2022), and (E) final assessment of Bakers Beach monitoring site indicating reduced sea spurge cover (April 2024).
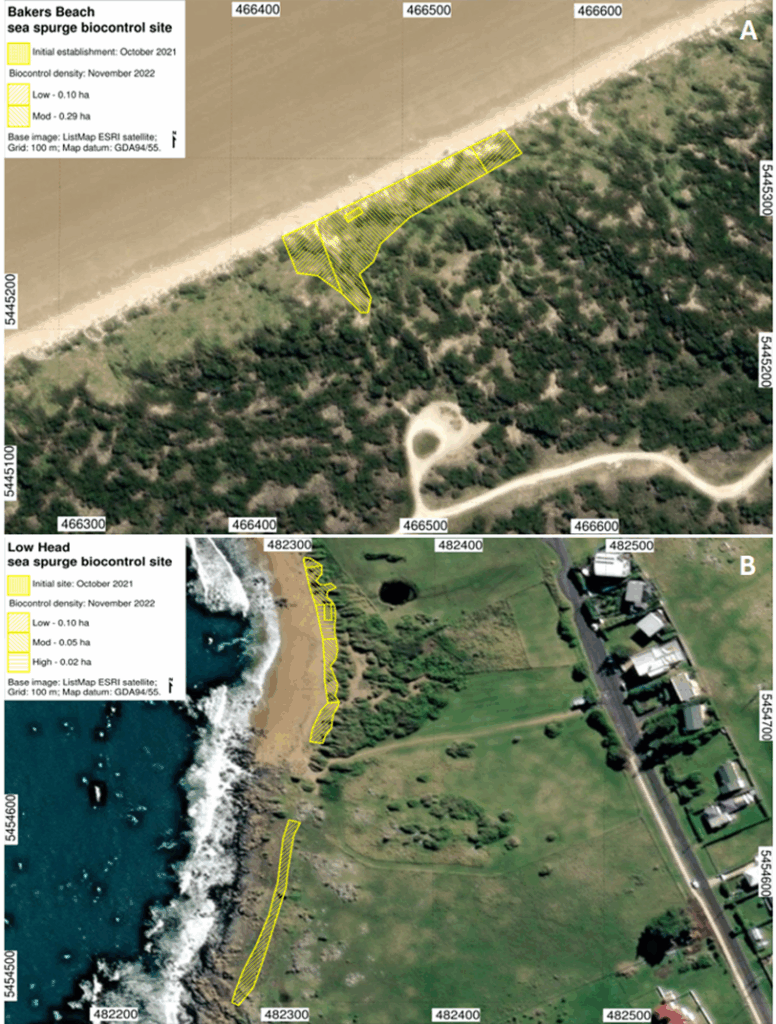
Figure 6. Natural spread of the sea spurge biocontrol agent, Venturia paralias, out of the dedicated monitoring sites to infect surrounding sea spurge plants in Tasmania at (A) Bakers beach, and (B) Low Head. Images prepared by Dr. Jon Marsden-Smedley (SPRATS).
Assessment of agent impacts on sea spurge populations in Victoria and Tasmania
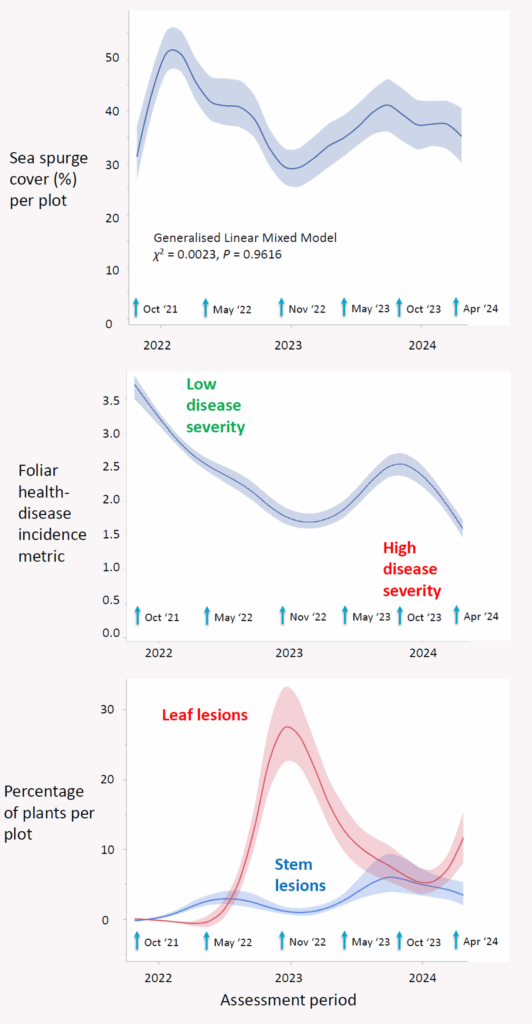
To evaluate the impact of the biocontrol agent V. paralias, the project team monitored changes in sea spurge cover, height, health, and disease symptoms across nine fixed monitoring sites.
Over the course of the project, average sea spurge cover declined from 45.9% to 37.4%, with the most notable reductions observed at Low Head in Tasmania (−20.2%) and Sand Island in Victoria (−17.4%). In contrast, plant height showed a slight upward trend, increasing from 34 cm to 38 cm.
Plant health, assessed using a five-point scale, declined steadily from an average score of 3.1 (healthy) to a level indicating poor health by the final assessment. The most significant declines were observed at Low Head, Sand Island, and London Bridge.
Disease symptoms, particularly leaf lesions, followed a seasonal pattern. Initially absent in Tasmania, leaf lesions became widespread from the second assessment onward and remained more prevalent at Tasmanian sites than Victorian ones—suggesting the pathogen is more active during milder spring conditions.
The statistical analysis conducted for this sea spurge biocontrol case study provides a compelling narrative of how the fungal pathogen V. paralias has established and impacted sea spurge populations across monitored sites in Victoria and Tasmania. These analyses were presented at the 23rd Australasian Weeds Conference 2024 (Brisbane, August 25-29, 2024). Detailed results can be found at this link (PDF here) and summarised below:
To evaluate the biocontrol agent’s effectiveness, the research team employed a Generalised Linear Mixed Model (GLMM) framework. This approach allowed us to analyse repeated measures across multiple sites and time points, accounting for spatial and temporal variability in the data.
One of the key metrics assessed was disease severity, which combined visual indicators of infection (leaf and stem lesions) with plant health status into a four-point scale. The GLMM revealed a statistically significant 53 % decline in foliar health over 2.5 years (χ² = 46.5770, P < 0.0001), indicating that the pathogen was increasingly affecting sea spurge vitality.
In contrast, when assessing sea spurge foliage cover, the model found no significant overall reduction across all sites (χ² = 0.0023, P = 0.9616), suggesting that while plants were visibly affected, cover remained variable and site-dependent. However, when we examined the relationship between disease severity and change in sea spurge cover over time, there was a significant association (χ² = 5.4300, P = 0.0198): i.e., sites with higher disease severity tended to show a decline in sea spurge cover, whereas sites with lower severity often experienced continued growth of sea spurge cover.
Together, these findings demonstrate that V. paralias has not only established successfully across all monitored sites but is also exerting measurable pressure on sea spurge populations, particularly in areas where infection severity is high. The results suggest that the biocontrol agent’s impact is accumulating over time and may lead to broader ecological effects with sustained release and spread.