How Does Tungsten Travel in Ore-Forming Fluids?
We combined atomistic simulation, in‑situ spectroscopy, and thermodynamic modelling to reveal how tungsten (W) actually moves in geological systems. The result is a clearer playbook for when W is mobile, and when it drops out.
Key Takeaways
- Don’t chase W–Cl: No stable W–Cl transport species in brines—refines geochemical targeting and avoids false positives.
- Focus on F-rich systems: W moves efficiently as W–F complexes in acidic to near‑neutral fluids; prioritise F‑bearing intrusions and alteration zones.
- Redox-sulfur context matters: In reduced, neutral–alkaline settings, W travels as thiotungstates; align sampling with expected alteration/IOCG-style footprints.
- Practical artefacts delivered: Formation constants for key W–F species, stability-field charts, and process envelopes for exploration, processing, and environmental prediction.
What we delivered
- Decision enablement: Target ranking criteria for F‑rich systems; process envelopes (pH/T/F) for solubility control; environmental mobility bounds in reduced vs oxidised regimes.
- Formation constants pack: Key W–F species (WO₃F, WO₃F₂²⁻, HWO₃F₂) ready for GWB/PHREEQC use.
- Stability-field charts: pH–aF and pH–fO₂ at 300 °C, showing when tungstate, thiotungstate, or W–F dominate.
IMPACT — What You Can Do Now
For exploration
- Focus on F‑rich systems: W moves efficiently as W–F complexes in acidic → near‑neutral fluids; prioritise F‑bearing intrusions/alteration where speciation windows indicate high mobility.
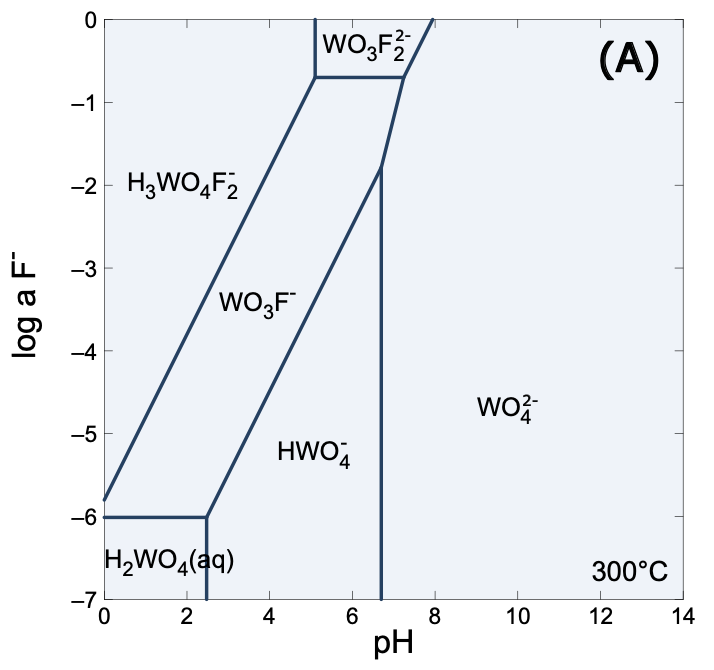
Stability fields of predominating tungsten species at 300 oC and water vapor-saturated pressure.
- Prioritise F‑bearing intrusions/alteration where speciation windows indicate high mobility.
- Avoid chasing W–Cl: Evidence shows no stable W–Cl transport species in brines.
- Use redox‑sulfur context: in reduced, neutral–alkaline settings W travels as thiotungstates; align sampling with expected alteration/IOCG‑style footprints.
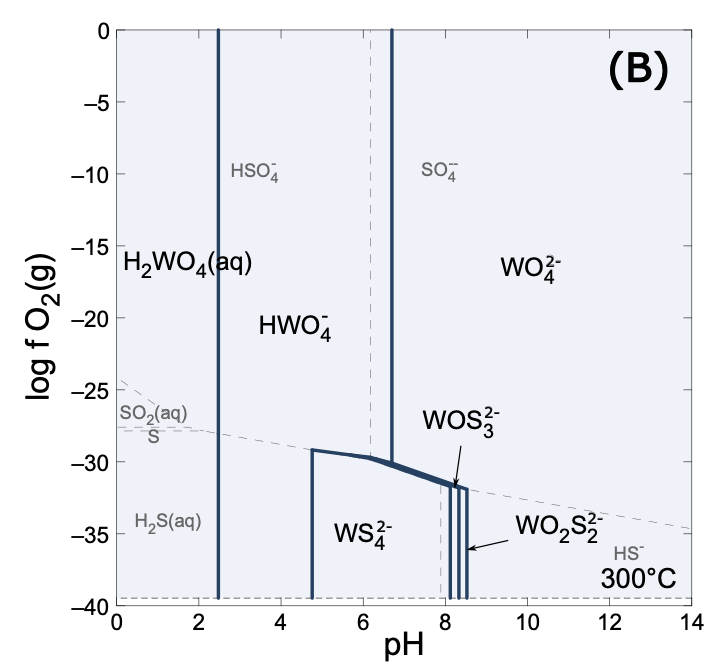
Tungsten speciation in sulfur-bearing solutions as a function of logfO2 and pH
For processing & geometallurgy
- Tune reagents: speciation windows indicate when fluoride increases W solubility; use this to set pH/T/F conditions for better extraction or to avoid unintended W carryover.
- Penalty‑element control: understanding W speciation helps anticipate deportment in circuits co‑processing Mo/REE‑rich feeds.
For environment / closure
- Predict W mobility: apply the delivered constants and diagrams to forecast W transport in sulfidic (thiotungstate) vs fluoride‑bearing waters under site conditions; supports risk assessments and monitoring plans.
How We Did It
- AIMD (ab initio molecular dynamics) to 600 °C / 2 kbar to identify stable W complexes in Cl‑, S‑ and F‑bearing fluids and their key bond signatures.
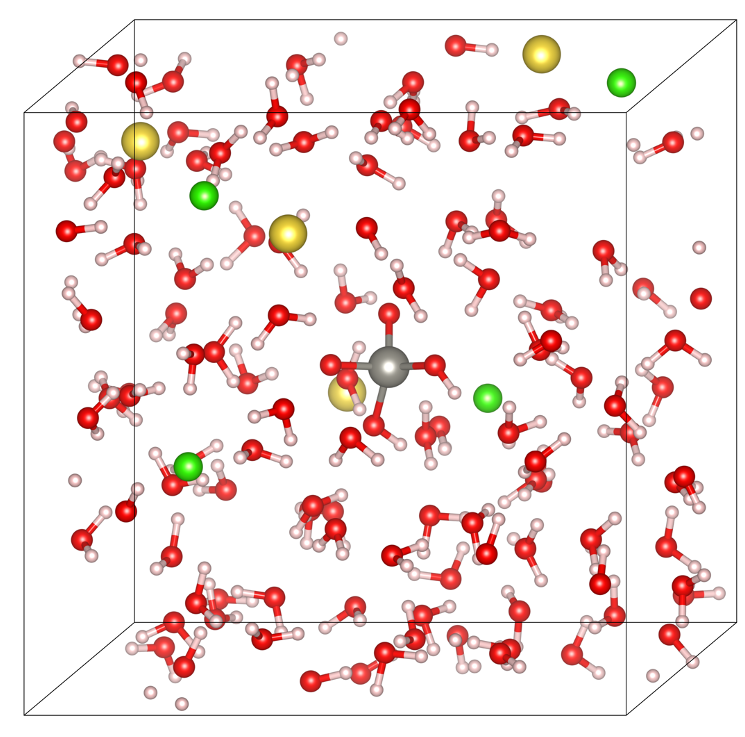
Snapshot of ab initio molecular dynamics simulation of tungsten in NaCl rich fluids
- In‑situ XAS (mAESTRO hydrothermal cell) experiment at Australian Synchrotron to verify coordination (O vs S vs F) directly in solution.
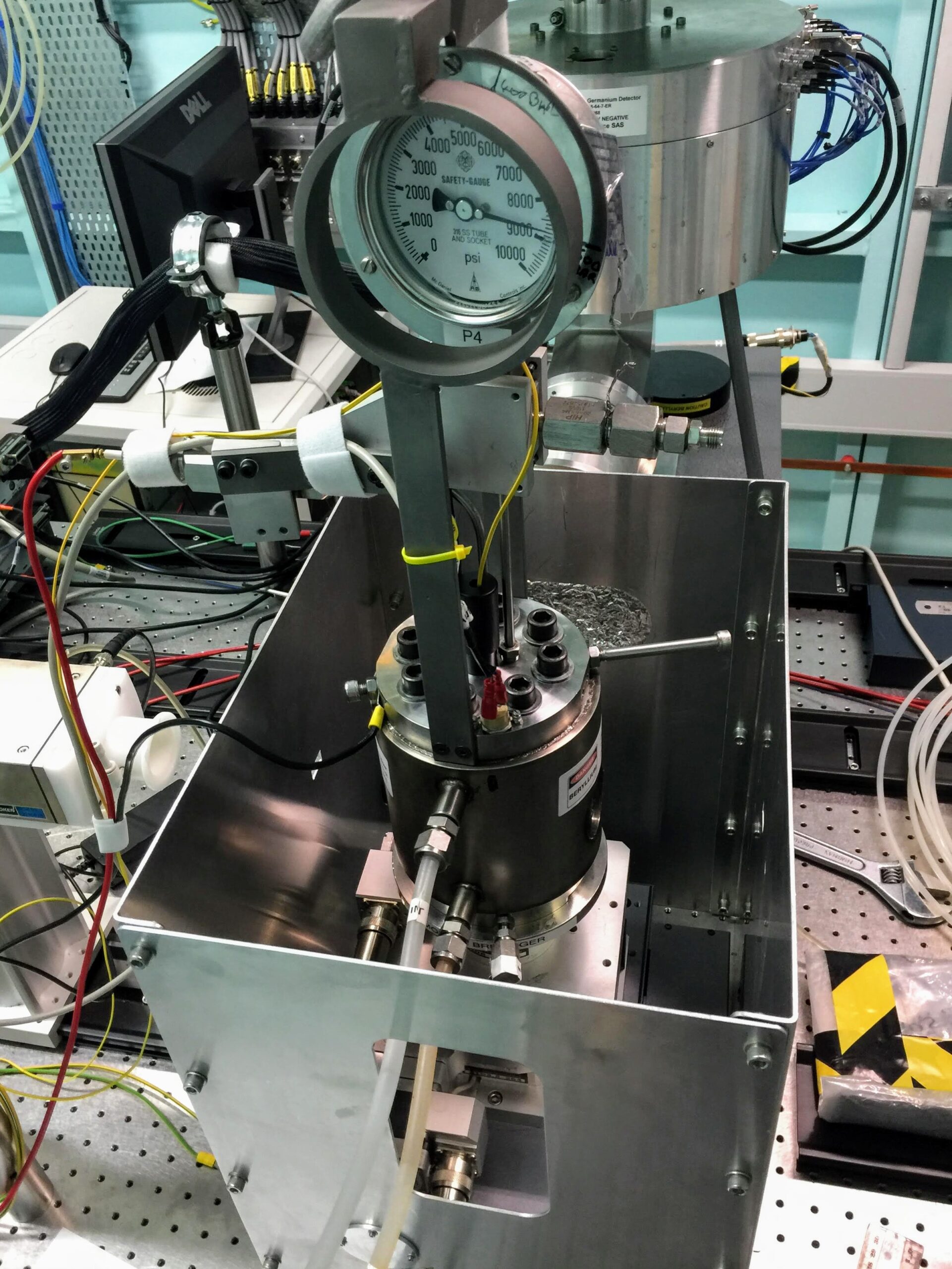
In-situ Synchrotron X-ray Absorption Spectroscopy (XAS) using mAESTRO cell, can heat up to 400oC, 500 bar
- Thermodynamic integration & modelling to deliver formation constants for W–F complexes and build stability fields (e.g., pH–aF at 300 °C) for targeting and process envelopes.
Tags: #Exploration #Processing #Environment #AIMD #XAS #Thermodynamics
Publication link
https://www.sciencedirect.com/science/article/pii/S0016703724003284
Previous post:
