How Germanium Sits in Zinc Ores: Experimental and Molecular Insights
We combined hydrothermal experiments, synchrotron microanalysis, and quantum chemical simulations to reveal how germanium (Ge) is incorporated into sphalerite in sediment-hosted Zn-Pb deposits. The result, published in Geochimica et Cosmochimica Acta in 2023, is a clearer playbook for critical metal enrichment and extraction:
Key Points
- Ge(IV) is the main form in sphalerite:
Germanium is incorporated as Ge(IV), bonded with reduced sulfur, both in experimental sphalerite and natural Zn-Pb ores from the MacArthur River deposit. - Multiple substitution mechanisms:
Ge(IV) can substitute for Zn(II) in sphalerite via charge balance by vacancies or by coupled substitution with other metals (e.g., Cu(I)), showing the crystal structure’s flexibility. - Experimental and natural samples agree:
Synchrotron XANES and SXRF mapping confirm similar Ge speciation and distribution in both lab-grown and natural sphalerite. - Implications for critical metal recovery:
Understanding Ge incorporation mechanisms helps guide exploration and extraction strategies for germanium as a by-product of zinc mining.
Background
Germanium is a critical metal, mainly recovered as a by-product from coal and zinc ores. Its distribution and incorporation in sphalerite (ZnS) are key to understanding resource potential and extraction. Sediment-hosted Zn-Pb deposits are major sources of Ge, which is enriched during hydrothermal processes.
Approach
- Hydrothermal Synthesis:
Sphalerite was synthesised under conditions mimicking sediment-hosted Zn-Pb deposits (200°C, water vapour-saturated pressure), using Ge(II) and Ge(IV) sources, with/without Cu and Fe.
- Microanalysis:
SEM, EBSD, EPMA, SXRF, and micro-XANES were used to map mineral phases and Ge speciation. - Quantum Chemical Simulations:
Density functional theory (DFT) was used to model Ge, Cu, and Fe substitution in sphalerite at the atomic scale.
Key Findings
Ge(IV) Incorporation:
We were able to probe the chemical state and local bonding environment of germanium within the sphalerite structure. Our results show that germanium enters sphalerite mainly as Ge⁴⁺, substituting for Zn²⁺. The figure below shows a synchrotron X-ray fluorescence image of a replacement rim of sphalerite and iron sulfide around calcite (A-C) and Ge-K edge X-ray absorption near edge spectra (XANES) collected (D).
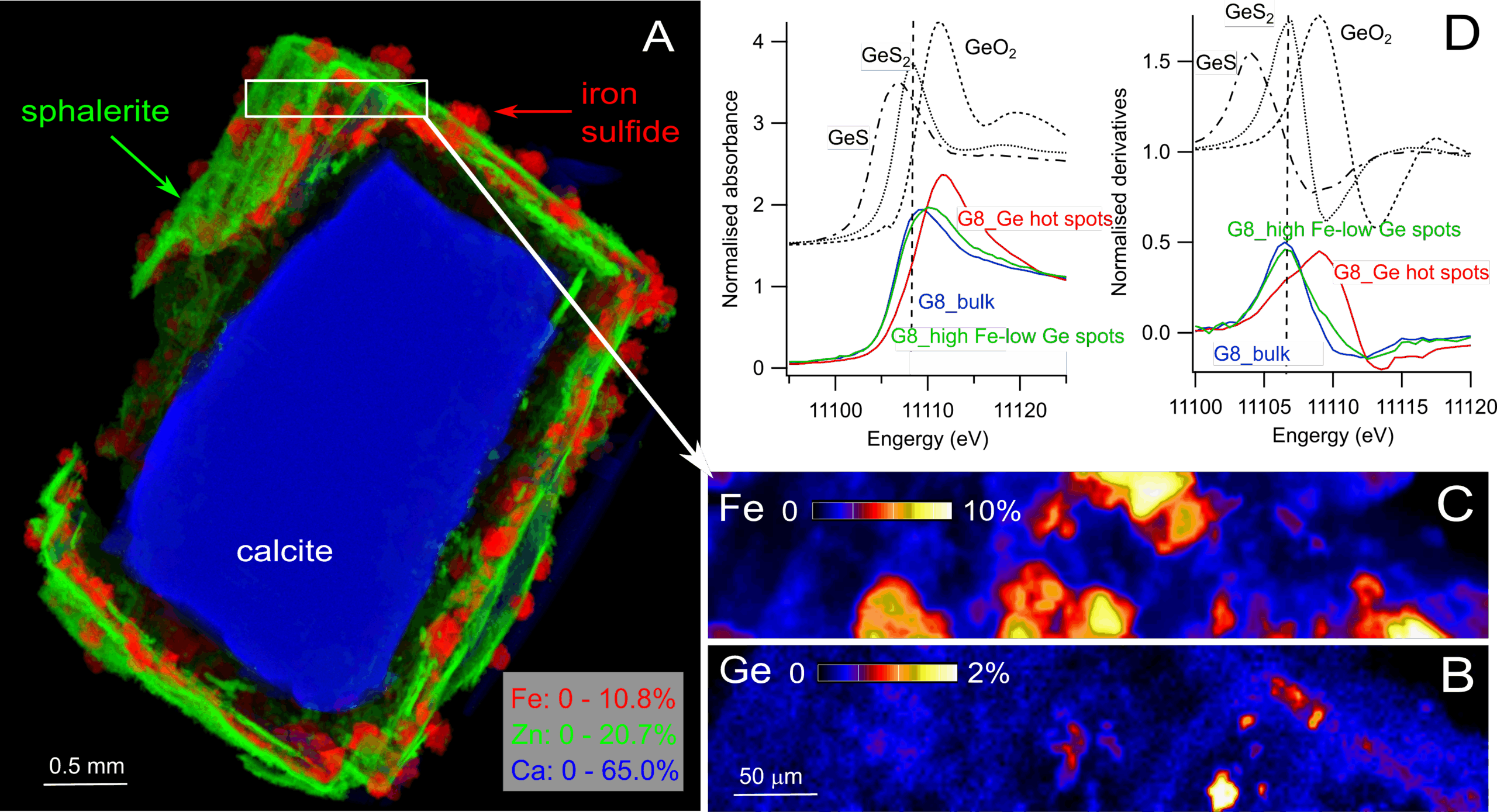
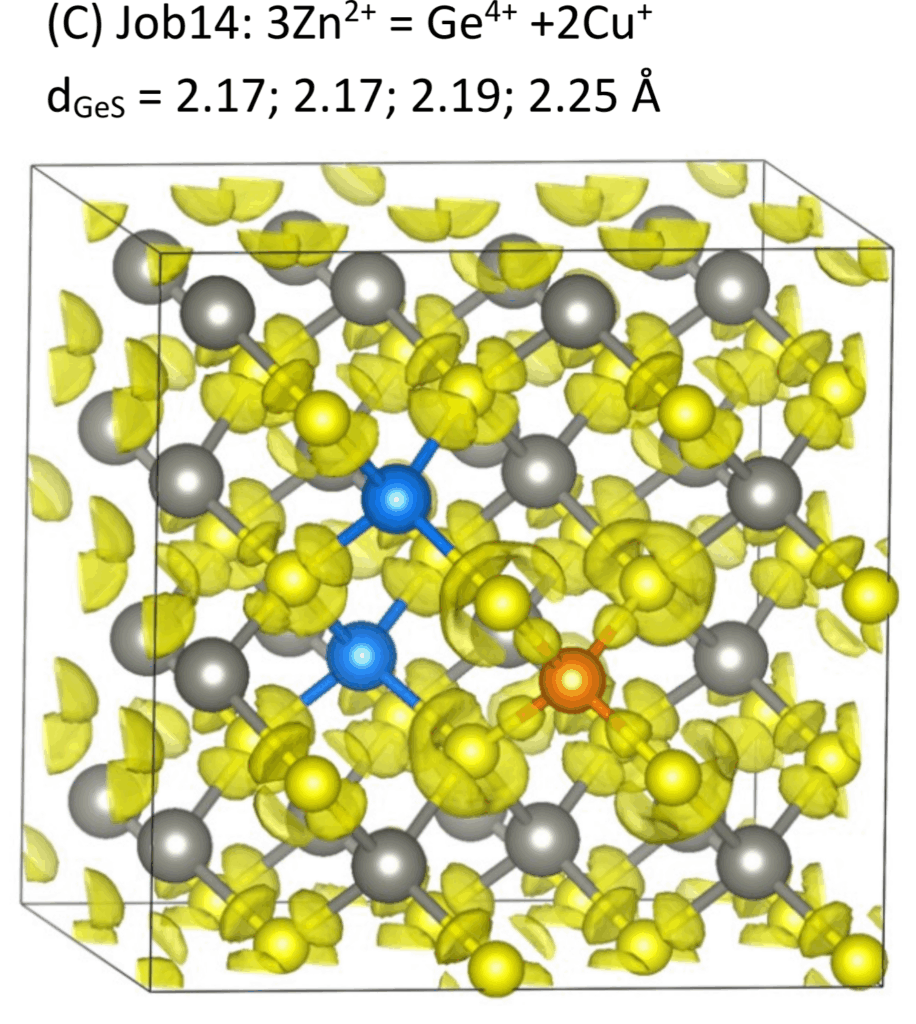
Substitution Mechanisms:
Surprisingly, our molecular simulation shows that the crystal structure can accommodate this charge mismatch either by minor local charge-balancing substitutions or even without strict compensation, indicating that sphalerite is more structurally flexible than previously thought.
Natural Ore Validation:
These findings help explain why natural sphalerites are enriched in germanium. We discovered that Ge(IV) speciation and distribution in sphalerite from the HYC Zn-Pb deposit in Australia match experimental results, confirming the relevance of lab findings.
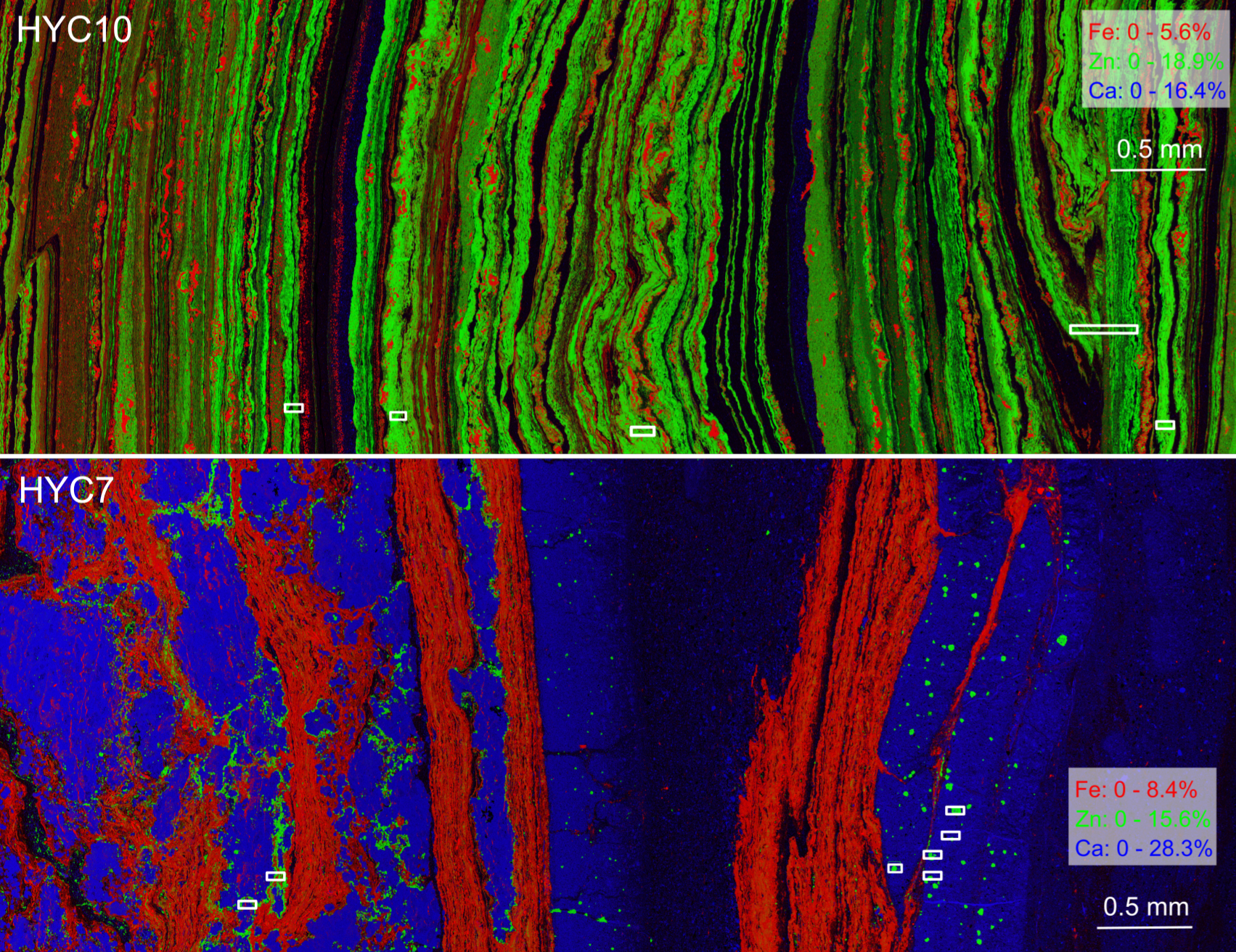
Impact and Significance
- Resource Assessment:
This study provides new insights into how critical metals are distributed in ore-forming systems – knowledge that could support future exploration for germanium-rich zinc deposits. - Extraction Strategies:
Insights into Ge substitution mechanisms inform targeted extraction and processing methods for germanium recovery. - Crystal Chemistry:
Demonstrates the resilience and flexibility of the sphalerite structure to accommodate Ge and other trace metals.
Practical Implications
- Exploration:
Focus on sphalerite-rich zones in sediment-hosted Zn-Pb deposits for Ge enrichment. - Processing:
Tailor extraction processes to account for Ge’s substitution mechanisms and speciation.
Tags: #Germanium #CriticalMetals #Sphalerite #ZnPbDeposits #Synchrotron #MolecularSimulation
Coming up next:
