DFT: Adsorbates on Mineral Surfaces for carbon mineralisation
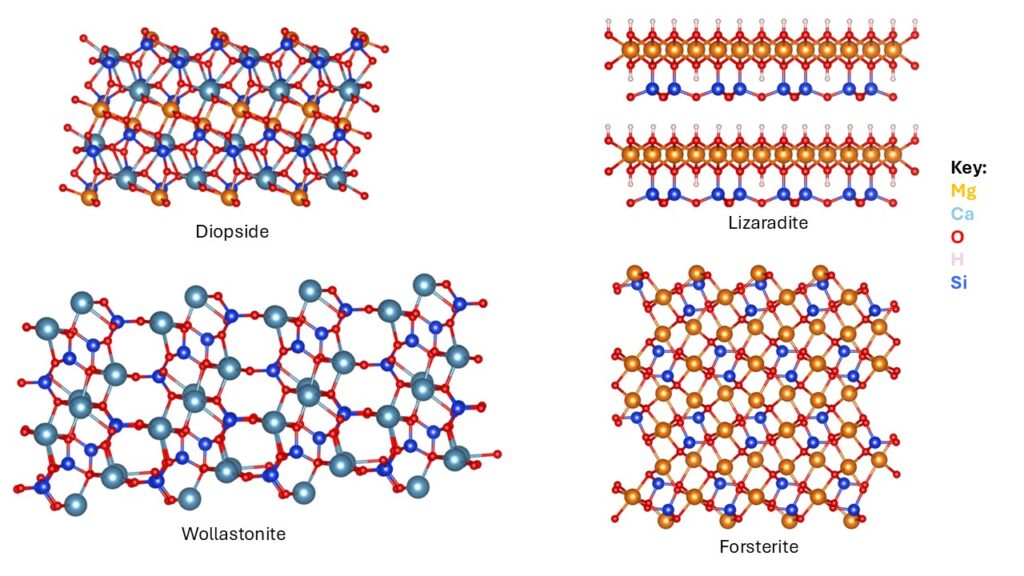
In this study we investigated the adsorption behaviour of key species—H₂O, CO₂, H₂CO₃, HCO₃⁻, and CO₃²⁻—on four minerals relevant to carbon mineralisation: wollastonite, diopside, lizardite, and forsterite. Adsorption is the first step toward carbonate formation, making it critical for understanding mineral reactivity and designing effective CO₂ sequestration strategies.
Background
Carbon mineralisation is a promising strategy for long-term CO₂ storage because it undertakes a reaction for form stable carbonate minerals. This process occurs naturally over geological timescales, but accelerating it requires a deep understanding of the fundamental steps involved. Adsorption of water and carbon species on mineral surfaces is the first critical stage before chemical reactions can form carbonates.
By studying adsorption energetics, electronic properties and reaction pathways we can:
- Predict mineral reactivity under different conditions
- Identify optimal minerals for CO2 sequestration
- Design processes which can enhance the natural carbonation cycle
- Reduce uncertainties in large-scale carbon capture and storage projects
Approach – Density Functional Theory (DFT)
- Mineral selection: Wollastonite, diopside, lizaradite and forsterite chosen for their relevance to carbon mineralisation
- Adsorbates Studied: H₂O, CO₂, H₂CO₃, HCO₃⁻, and CO₃²⁻
- Electronic Structure Analysis: Identify changes in charge and electronic properties upon adsorption, assess bond strengths
- Reaction Pathway: Performed Nudged Elastic Band (NEB) calculations for water and carbonic acid dissociation
Key Findings
- Carbonic acid dissociation is energetically more favourable than water dissociation across all studied minerals, proceeding without an activation barrier and releasing energy in all cases.
- The nature of the dissociation products depends on the mineral: bicarbonate forms on diopside, wollastonite, and lizardite, while complete dissociation to carbonate occurs on forsterite.
- Water dissociation is less favourable, with activation barriers on all but lizardite (where dissociation is not supported at all), and is only exothermic on diopside. On forsterite and wollastonite, water is more stable in its molecular form, as indicated by the endothermic reaction energies.
- These findings highlight the importance of mineral surface properties in governing reaction pathways and energetics for both carbonic acid and water dissociation, with implications for understanding mineral-fluid interactions in natural systems.
Practical Implications
- By identifying consistent trends in adsorption energies and electronic structure responses across silicate surfaces, it contributes to the advancement of scalable mineral carbonation technologies.
- This approach enables the rapid evaluation of mineral candidates beyond those directly studied, offering theoretical insights that support the selection of promising substrates for engineered carbon sequestration.
- This strategy can be used for other surface/material adsorption calculations where the electronic and physical properties want to be explored
