Copper in hydrothermal fluids
Copper mobility in hydrothermal systems depends on how Cu(I) complexes form, hydrate, and interact with other ions as fluids transition from dense brines to low-density vapours. Using advanced tools: ab initio molecular dynamics, in-situ spectroscopy, and geochemical modelling, we identified the key copper species under realistic geological conditions and quantified the impact of fluid density on hydration and charge balance. For the first time, we calculated stability constants (log K) for neutral NaCuCl₂(aq) and mixed-ligand species such as CuCl(HS)⁻, enhancing predictive models for ore formation and mineral processing.
Key Findings on Copper Speciation and Fluid Evolution
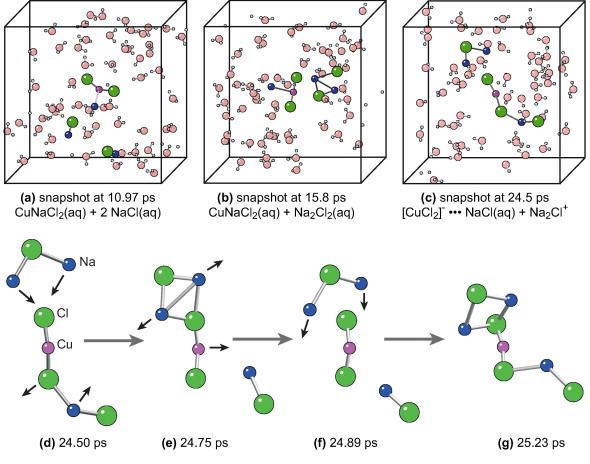
Cu in NaCl rich fluid at 1000 °C, 1500 bar (0.29 g/cm3) showing the formation of neutral NaCuCl₂(aq) species.
- Linear CuCl₂⁻ dominates in dense brines; CuCl₃²⁻ is negligible at high temperature
- In NaCl-rich brines from subcritical to supercritical conditions, Cu(I) occurs mainly as a distorted linear CuCl₂⁻
- CuCl₃²⁻ is minor at 25 °C and becomes thermodynamically unstable by 327 °C, consistent with XAS and molecular dynamics results.
- Charged linear complexes remain the primary copper carriers in high-density fluids.
- Mixed-ligand CuCl(HS)⁻ is highly stable at elevated temperatures
- Thermodynamic integration and MD yield log K ≈ 11.47 for CuCl(HS)⁻ at 327 °C.
- Activity maps show a broad stability field where CuCl(HS)⁻ dominates, reflecting ligand preference: HS⁻ > Cl⁻ > H₂S.
- Explains copper mobility in sulphur-bearing chloride fluids relevant to ore-forming systems.
- Neutral NaCuCl₂(aq) species emerge as fluid density drops
- At low densities (e.g., ρ ≈ 0.29 g cm⁻³ at 1000 °C, 1500 bar), CuCl₂⁻ associates with Na⁺ to form neutral NaCuCl₂(aq) and transient clusters.
- Na⁺ remains outer-sphere with short residence times (< 15 ps), explaining why EXAFS rarely detects a second shell.
- Neutral species become increasingly important in vapour-like fluids, influencing copper transport in late-stage hydrothermal systems..
- Hydration decreases linearly with density, driving complex stability
- MD shows hydration numbers for Cu⁺, Na⁺, and Cl⁻ decline linearly with decreasing fluid density (R² ≈ 0.98–0.99).
- Entropy gain from water release promotes ion association and neutrality at low density, underpinning density-dependent log K behaviour used in predictive geochemical models.
Geological implications
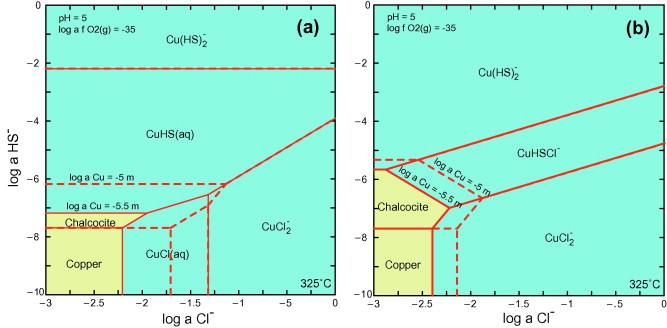
Comparison of mineral solubility and predominant aqueous Cu(I) species in the Cu(I)–Cl⁻–HS⁻–H₂O system at 325 °C, as a function of chloride and bisulfide activities. (a) Calculated using published formation constants from experimental studies; (b) Based on MD-derived formation constants for aqueous Cu(I) species. In fluids enriched in both Cl⁻ and HS⁻, the mixed-ligand complex CuCl(HS)⁻ dominates across a wide stability field, highlighting its critical role in Cu transport in hydrothermal systems.
- Phase separation and vapour transport
- During magmatic–hydrothermal phase separation, the liquid phase retains CuCl₂⁻.
- The vapour can still transport significant copper via neutral NaCuCl₂(aq), especially in alkali-rich systems.
- This explains copper partitioning into vapour observed in porphyry deposits.
- Supports the concept of vapour-driven pre-enrichment of shallow ore zones.
- Mixed-ligand stability in S–Cl fluids
- Ore-forming systems often contain both HS⁻ and Cl⁻.
- Strong stability and wide predominance of CuCl(HS)⁻ keep copper mobile across intermediate S/Cl ratios.
Prevents premature precipitation during fluid evolution. - When HS⁻ availability drops (due to fluid mixing, pH changes, or oxidation), speciation shifts back to CuCl₂⁻.
- This creates sharp solubility gradients and triggers copper deposition with sulphides such as chalcopyrite.
- Density as a master control
- Hydration decreases linearly with fluid density.
- Neutralisation via outer-sphere Na⁺ pairing becomes favoured as fluids ascend, expand, or boil.
- Predictive models should include density-dependent hydration, not fixed values.
- Improves accuracy of copper solubility estimates during ascent and boiling.
- Triggers for copper deposition
- Loss of alkalis (e.g., consumed during feldspar alteration) or increase in density (cooling, compression) reverses neutrality.
- Speciation shifts back to charged CuCl₂⁻, reducing copper mobility.
- Sulphur depletion through sulphide precipitation collapses the mixed-ligand field.
- Both processes cause abrupt solubility drops, concentrating copper in veins and breccia zones.
Tags: #Copper #Exploration #AIMD #XAS #Thermodynamics
Publication link
CuCl Complexation in the Vapor Phase: Insights from Ab Initio Molecular Dynamics Simulations
