Rural R&D for Profit round 4 project (2019-2023)
Achievements of Rural R&D for Profit round 4 project: Underpinning agricultural productivity and biosecurity by weed biological control
Pre-release monitoring of vegetation at selected future release sites of biocontrol agent(s)
The overall aim of vegetation monitoring at prospective biocontrol agent release sites was to characterise the population structure (e.g., plant density and size distribution) and abundance (e.g., biomass, foliage cover) of the focal weed and associated vegetation prior to release of a biocontrol agent. Pre-release monitoring establishes a baseline of vegetation condition against which the impacts of the biocontrol agent on the host weed can be assessed over time.
Between July and November 2020, 14 permanent monitoring plots (20 m × 20 m; 400 m2) were established across 7 sites in 3 regions in south-east Queensland, southern NSW and the ACT. We sampled a range of invaded habitats, including highly degraded and intermittently grazed remnant grassy Eucalyptus woodlands with an understorey of predominantly non-native pasture grasses, pine plantations, grazed pastures and roadsides. This will allow us to ascertain which, if any, habitat features are most strongly associated with African boxthorn population growth and, in the future, performance of the biocontrol agent(s) released. Within each plot, pre-release baseline data were gathered on African boxthorn population demographics using methods developed in April 2020 in the ACT.
Altogether (i.e. summed across the 14 plots), we identified, tagged and measured size and reproduction parameters for 258 plants. This data synthesis exercise revealed significant variation in estimated African boxthorn population productivity across the 14 plots, with a mean ± SD of 6.2 ± 5.4 (ranging from 1.2 to 18.7) tonnes per hectare. This variability will enable us to determine whether establishment, spread and impact of released agent(s) on African boxthorn are influenced by plant size, population density and other environmental variables.

Example of a plant that was destructively harvested by sawing basal stems at ground level, followed by an estimate of above ground wet biomass (kg) weighed using hand-held spring scales. Note the large mass of unripe (green) and ripe (red) fruit but lack of white-pink flowers, thus representing peak fruit output, based on field observations made across the ACT between October 2019 and April 2020.
During March 2021, 11 permanent monitoring plots (20 m × 20 m; 400 m2) were also established at different sites in semi-arid regions of South Australia, consisting of grazed grasslands and grassy woodlands, where African boxthorn invasion causes significant impacts on grazing productivity and the health of native ecosystems. Altogether, since sampling began in July 2020, we established a total of 25 monitoring plots (1 per site) throughout South Australia, south-east Queensland, southern NSW, and ACT, spanning a variety of climate and land use contexts – that is, cool temperate to subtropical to semi-arid grasslands and grassy woodlands used for beef cattle grazing.

Example of habitat context at four plots sampled in Queensland, ACT and South Australia.
We assessed the abundance of desirable vegetation associated with African boxthorn invasion within 16 of the 25 established plots, and found that the percentage cover of pasture grasses and herbs decreased significantly with increasing cover of African boxthorn, thus confirming previous observations that invasion by this weed can reduce the condition of desirable vegetation. The magnitude of decline was greatest for pasture grasses (5-fold decrease as African boxthorn cover approached 80 %) than herbs (2-fold decrease as African boxthorn cover approached 80 %). These data represent a baseline (i.e., pre-release of biocontrol agent) assessment of vegetation condition against which the benefits of biocontrol agent(s) can be evaluated in the future.
Approved release of the pathogen from biosecurity containment and establishment of a laboratory culture
The Department of Agriculture, Water and the Environment (DAWE) informed CSIRO in May 2021 that a preliminary assessment of the release application for the African boxthorn rust fungus Puccinia rapipes, which was submitted in November 2020, had been completed by a panel within the Department. The panel provided feedback and asked a series of questions. CSIRO answered DAWE questions and made the required minor revisions to the release application in early June 2021. In mid-July 2021, DAWE informed CSIRO that after having considered our responses and revisions they decided to proceed with finalising the preliminary draft risk analysis for P. rapipes and sending it to Plant Health Committee (PHC) for comment. CSIRO was told that all DAWE internal reviewers were comfortable with the draft recommendation to release P. rapipes based on outcomes of the risk analysis. DAWE sent the preliminary draft risk analysis in early August to the national Plant Health Committee for consideration and comments.
The fungal culture is being maintained on African boxthorn seedlings within plant growth facilities at CSIRO’s Black Mountain laboratories. Viable spores are being collected from infected leaves, then dried and frozen for long term storage.
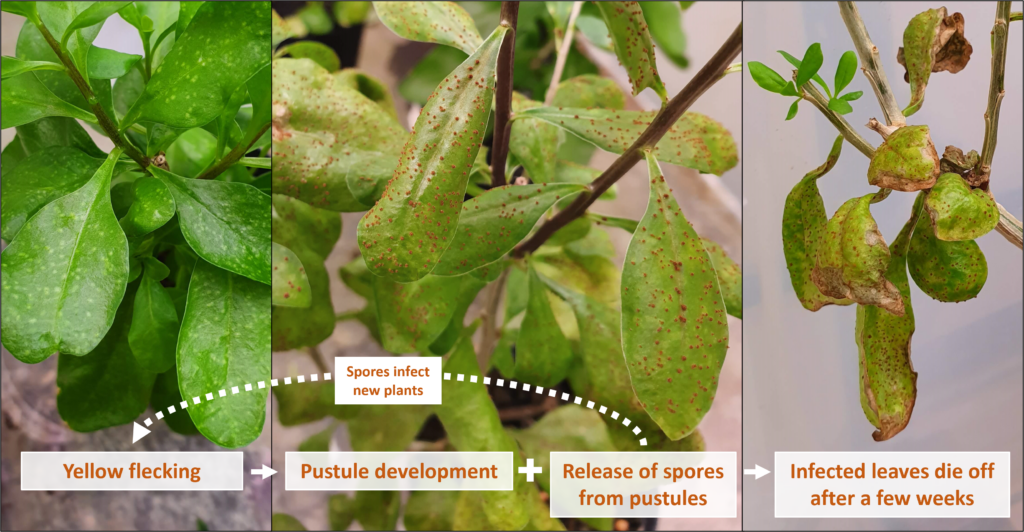
Depiction of disease progression for the fungus on Puccinia rapipes
Experimental optimisation of pathogen release methods in the field
Experimental releases of the fungus were undertaken on dense African boxthorn infestations in the field in the ACT, with the aim of optimising delivery methods to participating stakeholders. The experiments were undertaken between April and June 2022, testing viability of spores which had been removed from freezer storage for various lengths of time, and testing the time of day of release into the field. Upon removal from the freezer, spores were mixed in a 0.1 % Tween solution in a spray bottles, then immediately sprayed liberally across young, healthy foliage of host African boxthorn plants and covered with plastic bags for either 17, 20, or 24 hrs to retain local humidity for spore germination and infection. Post-release monitoring was undertaken after 2 weeks for both trials, with no signs of infection of the host African boxthorn by the fungus. For each of these two field experiments, fungal spores were tested for viability at the time of inoculation – and all batches of spores were found to be viable.

Method used to inoculate naturalised African boxthorn populations with the rust fungus Puccinia rapipes.
It is possible that infection did occur in the field, but cool autumn-winter temperatures in Canberra caused a delay in the onset of visible symptoms. Through a small field experiment set up in August 2022 in the ACT, it was confirmed that the fungus can remain dormant within the leaf tissue (for up to two months) until the ambient temperature become sufficiently high enough to induce disease symptoms.
Pathogen releases at fixed monitoring plots
The fungus was released using the spore suspension and stem-bagging method described above (with stems being bagged for 17 hrs) at two sites in the ACT and one site in QLD. Releases were made in mid-November 2022 once weather had become sufficiently warm (days regularly over 20°C).
At the ACT plots, post-release surveys of fungal infection on African boxthorn leaves were undertaken in February 2023, three months after the fungus was released. Observations were also made of infection on nearby control stems that had not been previously inoculated with the fungus. Altogether, 69 % of the inoculated stems comprised of leaves with visible symptoms of fungal infection, predominantly at the telia stage of reproduction. Some control stems also bared infected leaves, indicating secondary infection whereby the fungus had completed its lifecycle on the first set of inoculated stems that in turn produced spores that spread to nearby stems. As such, it is likely that the fungus has now become established at these sites and is beginning to spread within the local Africa boxthorn population. At the QLD release plot, branches were checked for infection after about 5 weeks, and all branches (100%) had some level of infection present, ranging from a few scattered pustules on a couple of leaves to most leaves covered in infection. After 9 weeks, branches were checked again, and infection was still present on most stems.
Ongoing monitoring will reveal the rate at which the fungus spreads through these local areas.
Community engagement in a pilot mass-release program of the pathogen across Australia
From September 2022 until February 2023, CSIRO researchers launched a pilot biocontrol agent release program in partnership with weed management stakeholders, with the aim of trialling the optimised release protocols for the rust fungus in a variety of landscapes and climate contexts. We selected participants equitably to reflect the broad range of site conditions and across the range of African boxthorn’s core distribution in south-eastern Australia from southern Queensland through to the Eyre Peninsula in South Australia.
Registered participants were sent a biocontrol agent release kit consisting of a vial containing 0.1 ml dried spores of the rust fungus, materials to prepare the agent for release, and a comprehensive set of release and monitoring instructions.
Altogether, 126 biocontrol agent release kits were distributed to 33 participants, drawn from 20 organisations, throughout Queensland, NSW, Victoria, and Tasmania. We also registered participants from Western Australia but permission to import the biocontrol agent to that state had not been granted until December 2022, after which time the daytime temperatures were too high to support fungal infection in the field.
Participants detected the fungus at ~43 % of monitored sites, however many sites had boxthorn plants that were severely stressed due to recent hot/dry weather and had dropped all their leaves – this usually occurred in sites in South Australia. Sites in Queensland appeared to have the most success, with 100 % of QLD sites monitored showing disease symptoms.
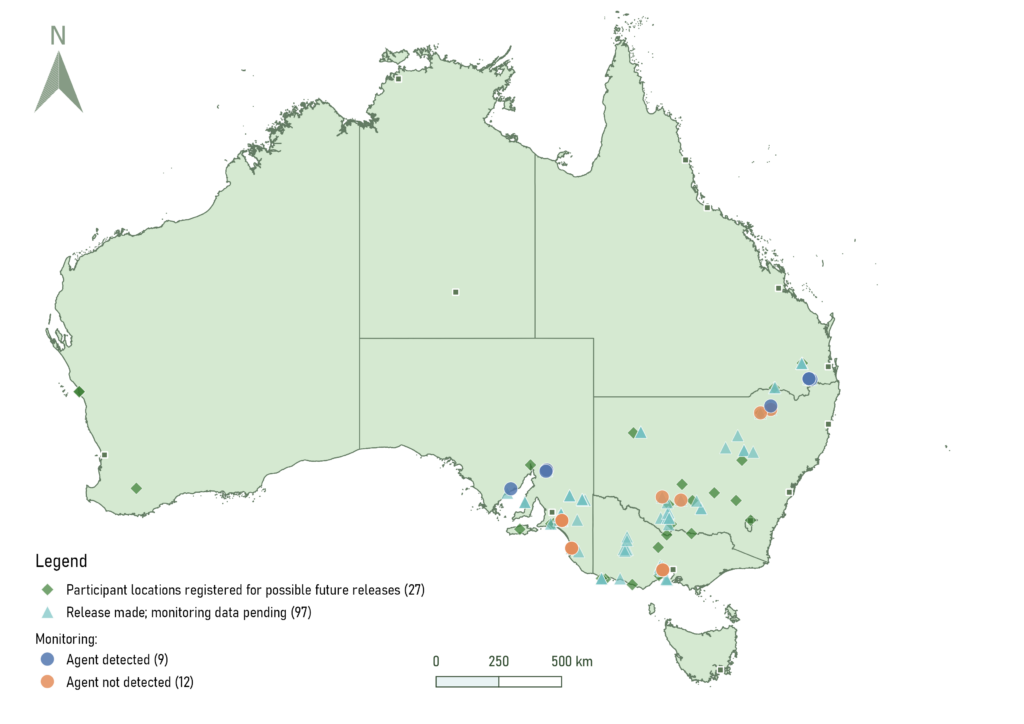
Sites which have been registered for future Puccinia rapipes releases (green diamonds) or have had release made but data on establishment is pending (blue triangles). Monitoring sites where the agent has been released and detected (blue circles) or not detected (orange circles).
Importation of a new candidate agent into quarantine to establish a colony and commence testing
As reported in the final report of the RRnD4P Round 2 project, a new accession of Cleta eckloni was imported to the CSIRO Quarantine facility located in Brisbane from the Western Cape province of South Africa in February 2020. After exporting C. eckloni to Australia, the remaining culture at the Centre for Biocontrol (CBC) at Rhodes University was starting to recover. In March 2020 national lockdowns were imposed in South Africa due to COVID-19. Mid-August 2020 was the first-time CBC staff were allowed to access the mass rearing and research facility. Some plant material had survived the period without maintenance but none of the insect colonies had survived. When domestic travel was permitted, CBC staff commenced field surveys to re-collect samples of C. eckloni, N. serietuberculata and C. distinguenda to restart laboratory colonies. Due to these efforts’, candidate agents were available for import once logistics chains were available.
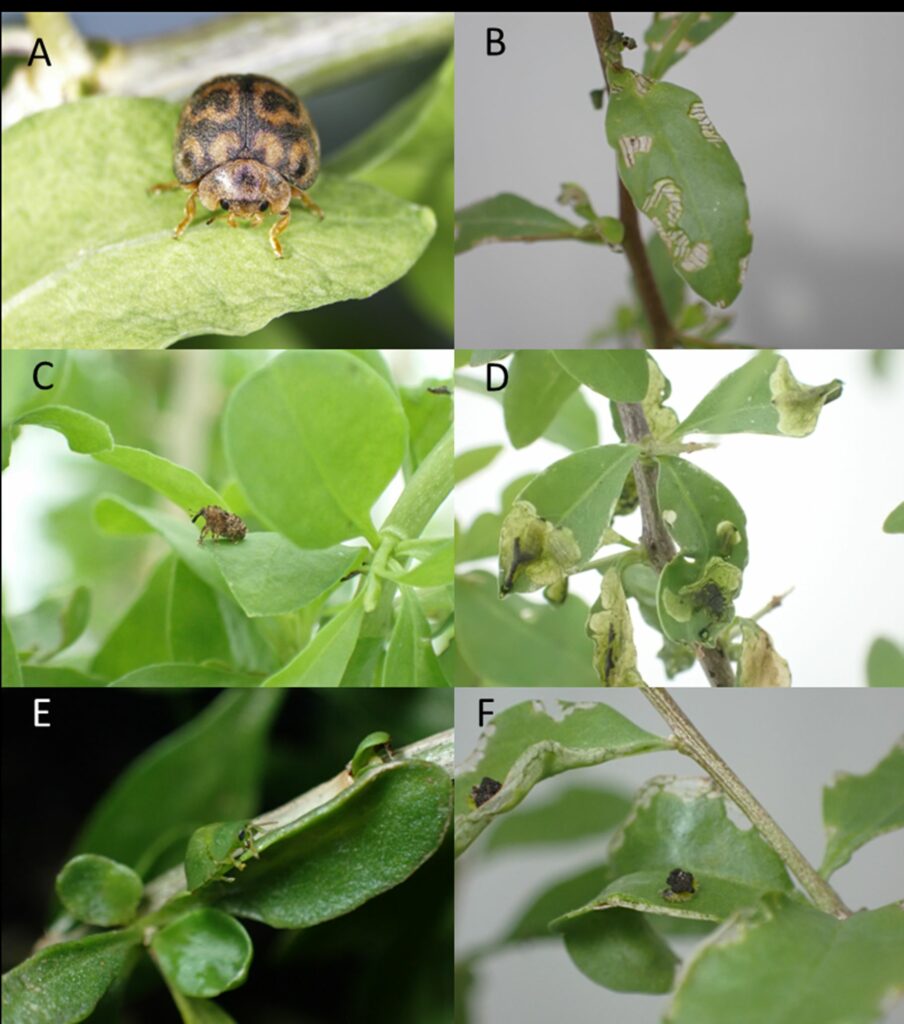
Photos of each of the insects and prioritised for host specificity testing in Australian quarantine. A) Cleta eckloni, B) associated Cleta eckloni feeding damage on African boxthorn, C) Neoplatygaster serietuberculata, D) associated Neoplatygaster serietuberculata larval feeding damage on African boxthorn, E) Cassida distinguenda, F) associated Cassida distinguenda feeding damage on African boxthorn.
A further importation of Cleta eckloni (Western Cape) from South Africa was then attempted in January 2021. Unfortunately, due to delays in logistic transport chains, all insects died in transit. Neoplatygaster serietuberculata was consequently prioritised for further importation attempts as it is more resistant to starvation relative to C. eckloni and thus able to withstand extended freight periods. Several importations of this insect were made in March, May and June 2021. A culture of this insect was maintained in the quarantine facility from March 2021 until November 2022. A third insect Cassida distinguenda was imported into the Brisbane quarantine facility in April and June 2022. A culture of this insect was sustained until January 2023.
Host-specificity testing under quarantine of new imported candidate agents
Neoplatygaster serietuberculata – No-choice testing
The test list comprised of L. australe, which is an Australian native that is distributed across the southern half of Australia, and L. barbarum, L. chinense and L. ruthenicum which are all ornamental and of East Asian origin (Levin et al. 2007). Physalis peruviana and Lycianthes rantonetti, which are ornamentals just outside of the Lycium genus, were also included in a single no-choice test replicate. Both adult and larval feeding were assessed, along with the number of eggs and the number of leaves oviposited on, the number of larvae observed, and the new adults produced were recorded over the duration of the tests.
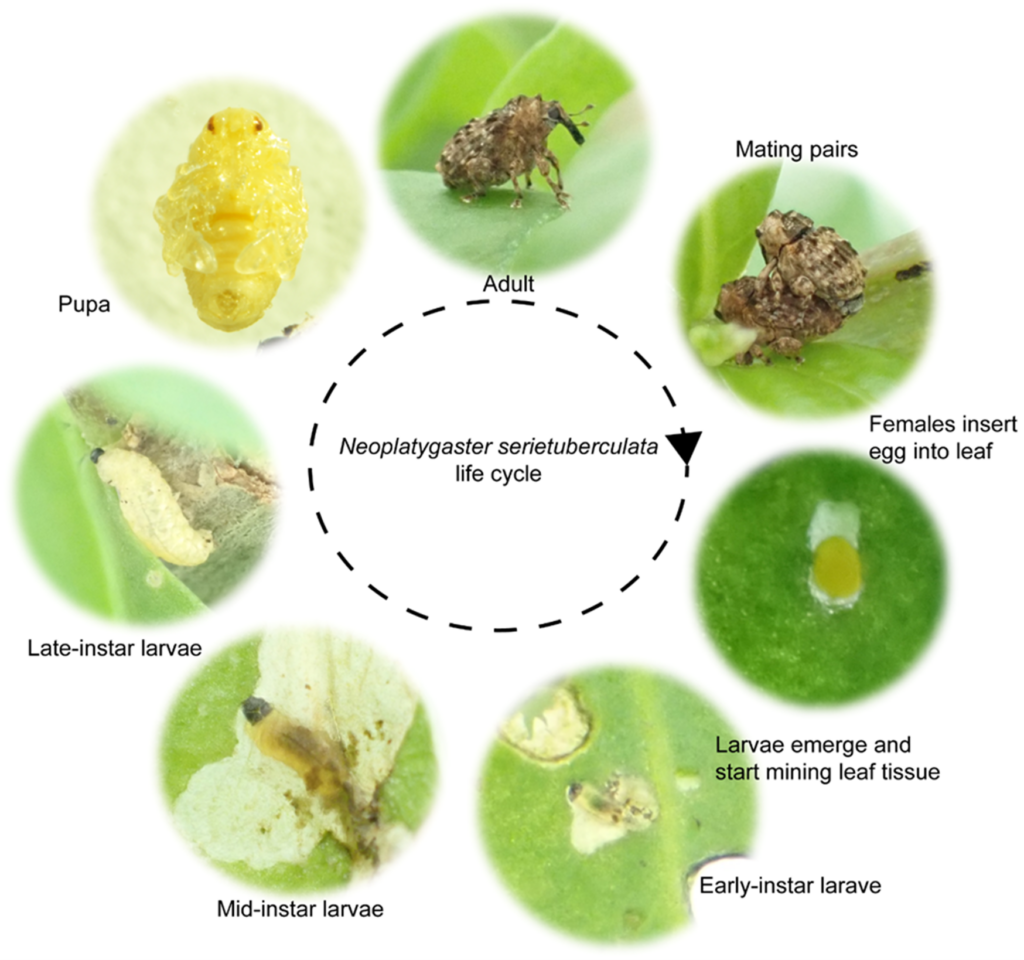
Biology and life cycle of Neoplatygaster serietuberculata, as determined by collaborators at Rhodes University.
Larval feeding in no-choice tests was highest on the native L. australe, followed by L. barbarum and African boxthorn, but there was no significant difference in observed feeding between plant species. Adult feeding was recorded across more of the test plant species, with significantly higher feeding recorded on the native L. australe. The highest number of adults was produced from tests on the focal weed African boxthorn, followed by L. australe and L. barbarum.
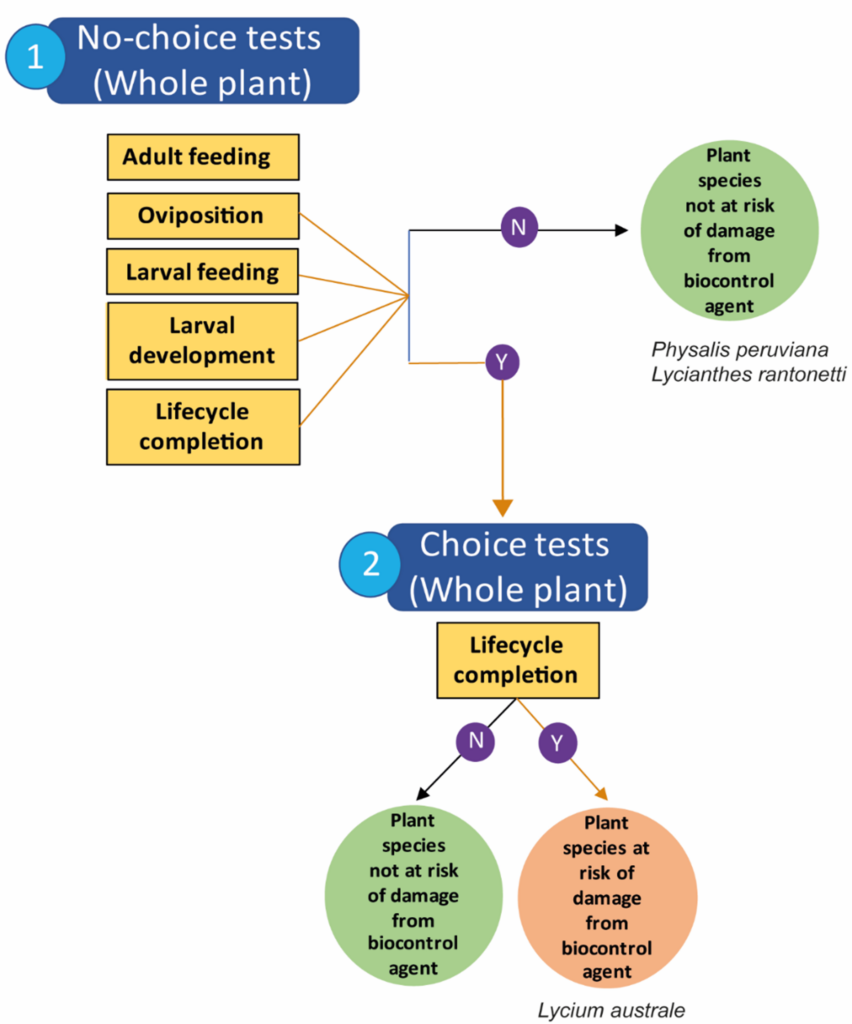
Decision tree for Neoplatygaster serietuberculata biocontrol risk.
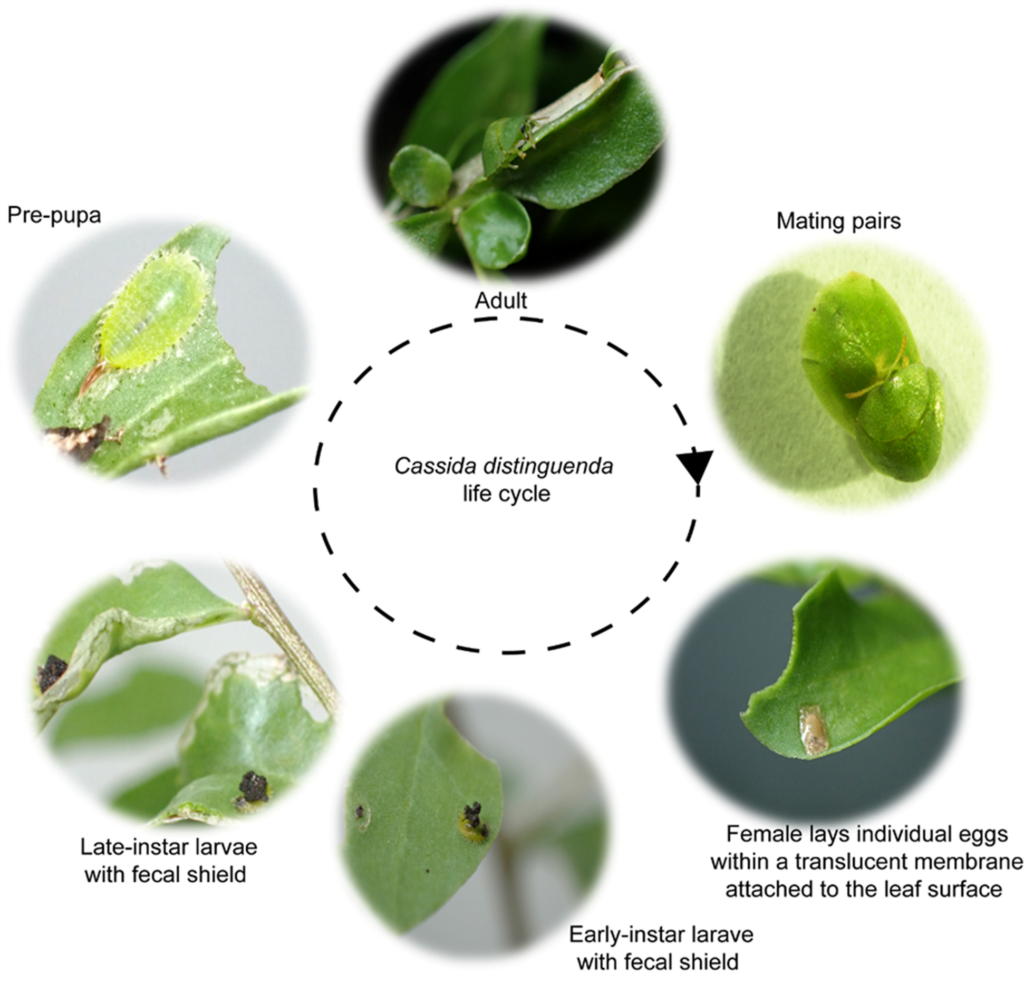
Cassida distinguenda – No-choice testing
No-choice tests were performed for L. australe and African boxthorn. Adult insects were placed on each plant of each species after which feeding, survival and oviposition were recorded for two weeks.
Biology and life cycle of Cassida distinguenda, as determined by collaborators at Rhodes University.
No choice tests revealed that feeding on the Australian native L. australe was higher than that observed on the focal weed African boxthorn for both adults and larvae, but not significantly so. The mean number of adults produced on African boxthorn was significantly higher than L. australe.
Adults produced during no-choice tests were then placed on the same plant species they were produced on for another generation to see if a persistent population could be maintained on L. australe and African boxthorn controls. Feeding, survival and oviposition were recorded for two weeks.
Feeding scores for C. distinguenda adult and larvae remained high across both L. australe and African boxthorn in the continuation trails. More adults were produced on the African boxthorn controls than that observed on L. australe, but the results were not significantly different, indicating that the Australian native is capable of sustaining C. distinguenda in the laboratory context over consecutive generations.
Cassida distinguenda – Choice tests
Paired choice tests of C. distinguenda adults on African boxthorn and L. australe were conducted Inside each cage, the two plants (African boxthorn and L. australe) were placed 10 – 15 cm apart from each other. newly emerged adults were placed on a petri dish in the middle of the cages. The location of the insects within the cage was recoded as well as feeding damage, oviposition, and the appearance of early and late instar larvae were observed for three weeks.
Adult C. distinguenda were located on both African boxthorn and L. australe during paired choice tests. Adult and larval feeding across both African boxthorn and L. australe was not significantly different. Early and late instar larvae were observed on both African boxthorn and Lycium australe.
Based on the consistent feeding damage and reproduction on the native non-target plant L. australe across no-choice, continuation, and choice tests, C. distinguenda was deemed to not be sufficiently host specific to pass a risk assessment for release.
Neoplatygaster serietuberculata – Paired choice tests (South Africa)
Lycium australe was shipped from Australia as bare rooted material to the quarantine facility at Rhodes University South Africa. Paired choice tests of N. serietuberculata adults on African boxthorn and L. australe were conducted in a quarantine facility at Rhodes University. Inside each cage, the two plants (L. ferocissimum and L. australe) were placed 10 – 15 cm apart from each other and a cardboard sheet was placed equidistant from the two plants to form a bridge that the insects could walk over from one plant to the other. At the beginning of each test, adult insects were placed at the centre of the bridge in each cage to enable the insects to orient themselves to the tested plants. The location of the insects within the cage was recorded after one hour from release. Consequently, daily recordings of location, feeding damage, and oviposition were taken for three weeks.
Adult N. serietuberculata were located on both African boxthorn and L. australe during paired choice tests during the 35-day exposure period. Adult feeding was recorded across both African boxthorn and L. australe. Oviposition occurred on African boxthorn and on L. australe, and eggs laid on L. australe were able to complete development.
Based on the high levels of feeding damage and reproduction on non-target plants in the Lycium genus, especially the native L. australe, no further testing was conducted as the insect was deemed to be not sufficiently host specific in the Australian context.
Decision tree for Cassida distinguenda biocontrol risk.
Provide a catalogue of additional candidate agents for African Boxthorn control
Native range surveys for potential biological control agents were conducted in South Africa by the staff and students of the Centre for Biological Control (CBC) at Rhodes University. Taxonomic and phylogenetic aspects of L. ferocissimum in both its native and invaded distributions were identified to ensure that potential agents were only collected from L. ferocissimum plants. More than 96 morpho-species of leaf, fruit and flower feeding arthropods were collected from over 50 sites across South Africa between 2017 and 2020. Among the insects there are 32 species of leaf feeders (Family (number of species): Chrysomelidae (11); Coccinellidae (4); Coreidae (1); Curculionidae (3); Cicadellidae (1); Coccoidea (1); Membracidae (2); Miridae (2); Pentatomidae (2); Pyrrhocoridae (1); Homoptera (1); Thripidae (2); Eriophyidae (1)), 3 species of fruit feeders (Tephritidae (2); Coreidae (1)), 1 species of seed feeder (Pyrrhocoridae (1)) and 5 species of flower feeder (Cerambycidae (1); Coccinelidae (1); Malachiidae (1); Melyridae (2)). Since then, nine stem inhabiting insects have been collected from sites in the Western cape Province. Taxonomic identifications of most collected insects were confirmed by the Plant Protection Research Institute (PPRI) Biosystematics Division – Insect Identification Service in South Africa. However, due to lack of appropriate taxonomic expertise in some insect families, many specimens still remain to be identified to the species level.
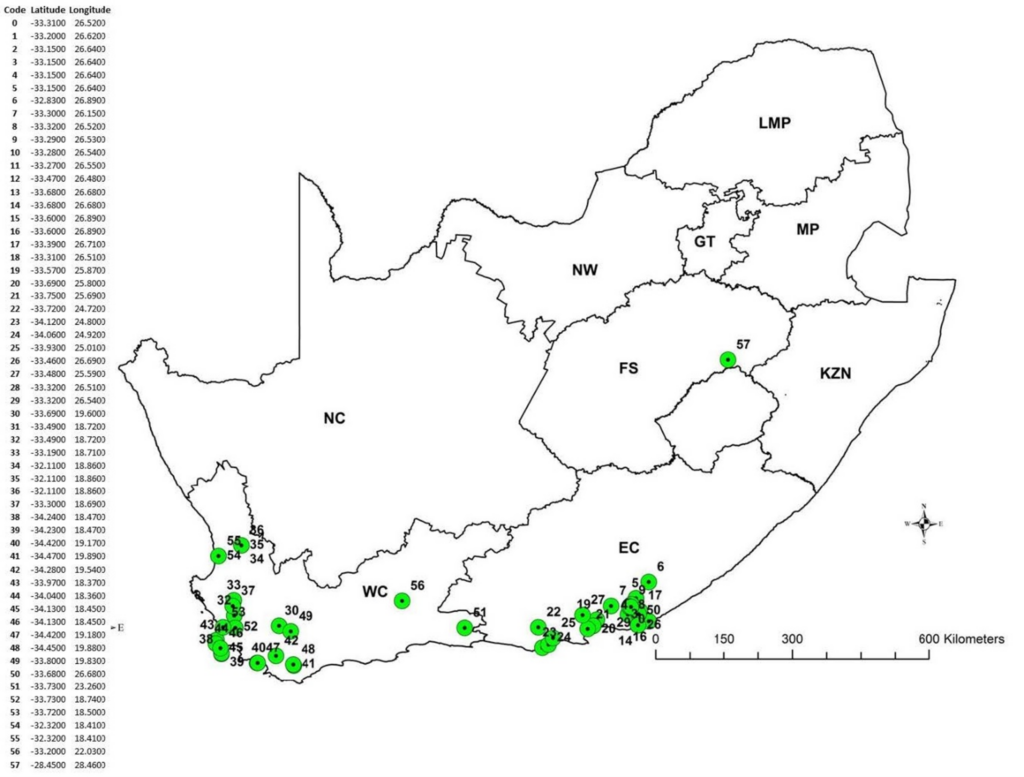
African boxthorn sites surveyed in South Africa by Centre for Biological Control. Associated site numbers and GPS co-ordinates are provided in the column left of the figure.
