Rural R&D for Profit round 2 project (2016-2020)
Achievements of Rural R&D for Profit round 2 project: Biocontrol solutions for sustainable management of weed impacts to agricultural profitability
Undertake a literature review on taxonomy and distribution of African boxthorn and known natural enemies of the weed in the introduced and native ranges
Taxonomy
Species within the genus Lycium are highly plastic, so delimiting and identifying species within the genus with morphological characters can be difficult (Levin et al. 2006; Venter 2000). This is especially relevant to regions like southern Africa that has a high diversity of species in this genus. Morphological variation within Lycium ferocissimum is substantial (Venter, 2000), perhaps influenced by the broad range of climatic and environmental conditions across its distribution in South Africa. Identification of this species in the field is therefore challenging.
Morphometric analyses across L. ferocissimum and other Lycium species in South Africa were undertaken during this project and this research is now published in McCulloch et al. (2020). No leaf or floral characteristics unique to L. ferocissimum were identified, making morphological identification of the species problematic. This is not an issue in Australia, because there are only four other Lycium species present, outside of cultivation, all with restricted distributions: the native L. australe and the naturalised L. barbarum, L. chinense, and L. afrum of Eurasian origin. To ensure the correct Lycium species was surveyed for candidate biocontrol agents, L. ferocissimum individuals for which the identity was confirmed with genetic analyses, were permanently tagged.
Distribution
In Australia, L. ferocissimum is widespread in coastal to semi-arid inland habitats and islands of southern Australia, with records from every jurisdiction (GBIF.org, 24th July 2018; Fig. 1). It is found predominantly in the southern part of the Australian continent in coastal and island situations (except Queensland). Inland, L. ferocissimum is abundant in areas of New South Wales, Victoria and South Australia, where it is a common weed of semi-arid pastures and rangelands and is often found growing along dry stream beds. It has a lesser, but significant presence in south-east Queensland, southern Western Australia, and Tasmania.
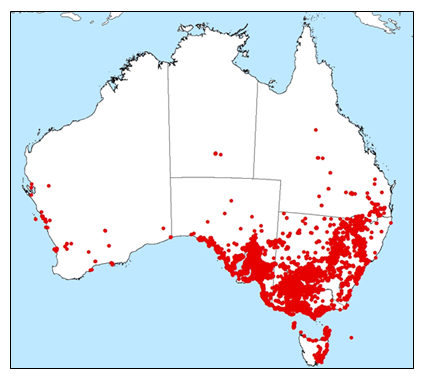
Figure 1. Current distribution Lycium ferocissimum (African boxthorn) in Australia (GBIF.org 24th July 2018).
Lycium ferocissimum has a relatively widespread distribution in South Africa (Venter 2000); however, most herbarium records are from the Eastern and Western Cape provinces. Its distribution overlaps with several morphologically similar and closely related species with which L. ferocissimum hybridizes.
Natural enemies
A comprehensive review of the literature was undertaken in late 2016 to identify potential agents already recorded on L. ferocissimum in the native range, South Africa, and determine if any of these are already present in Australia. At the time of the review, 4 fungi and 13 insects had been recorded on L. ferocissimum in Australia. None of these have been recorded in South Africa. We found records of only one pathogen, the rust fungus Puccinia rapipes, and eight insect species on L. ferocissimum in South Africa. Among these, the rust fungus, tortoise beetles, Cassida distinguenda, Cassida lycii and Cassida sp., and the mirid bug, Schuhistes lekkersingia appeared to be specific enough to warrant further study as potential biocontrol agents.
Defined goals for management of African boxthorn
In order to better understand the impacts of L. ferocissimum on Australian natural ecosystems and agriculture, and to guide the selection of candidate biocontrol agents, a national online stakeholder survey was conducted. Agricultural, community and environmental stakeholders were invited to complete a survey entitled ‘African boxthorn management objectives’, using the online survey platform SurveyGizmo between May and July 2017. Results from this survey are published in Ireland et al. (2019b).
Of the 239 responses received, respondents primarily identified as natural resource managers, specialists or extension officers (61%). Community, conservation or other interest group members (22%), agricultural landholder or graziers (19%), and agricultural land managers, agronomists or extension officers (7%) made up the remaining respondents, with some respondents identifying with more than one group. A range of reasons were provided by respondents as to why L. ferocissimum was problematic, aside from it being difficult (79% agricultural and 86% environmental) and costly (78% agricultural and 84% environmental) to control in both the agricultural and environmental sectors (Ireland et al. 2019b).
Many respondents (79%) agreed that biocontrol solutions for L. ferocissimum would be useful, particularly in difficult to access and environmentally sensitive areas, and when adopted as part of an integrated weed management framework. Respondents’ top six management objectives to which biocontrol needs to make a significant contribution were a reduction in:
- incidence of new infestations,
- management costs,
- negative impacts on native biodiversity,
- weed seed bank over time,
- the need for follow up manual control,
- herbicide use.
Nominating African boxthorn as a biocontrol target
The Invasive Plants and Animals Committee (IPAC; now the Environment and Invasives Committee; EIC), a cross-jurisdictional sectoral sub-committee of the National Biosecurity Committee, endorsed L. ferocissimum as a biocontrol target in August 2016. Unbeknownst to us, the nomination had been prepared and submitted to IPAC by DPIPWE, Tasmania prior to the commencement of the project. One of the members of the project team, however, approached the authors of the nomination document to assist in updating and reformatting the information into a review paper on L. ferocissimum for publication (Noble et al. 2021).
Conducting genetic analysis on samples of African boxthorn from different regions in Australia and the native range
Putative L. ferocissimum (i.e. tentatively identified morphologically in the field) samples were collected across its native range in South Africa and introduced range in Australia. A first set of genetic analyses (three chloroplast markers, one nuclear marker) were conducted in order to: (a) confirm plant identity, (b) assess genetic structuring across the native and invaded ranges (to explore the provenance of the invasive lineage), and (c) assess evidence for hybridisation between L. ferocissimum and other Lycium species that occur in Australia. Results from this study are published in McCulloch et al. (2020).
All samples collected in Australia were confirmed as L. ferocissimum, with no evidence of hybridisation with any other Lycium species. Ten samples from South Africa putatively identified in the field as L. ferocissimum were genetically characterised as different (unidentified) Lycium species. Nuclear and chloroplast genetic diversity within L. ferocissimum across both South Africa and Australia was low, with no evidence of genetic structure. The lack of any detected genetic diversity and structure across L. ferocissimum in South Africa made inferring the introduction history of the invasive lineage challenging. However, one of the two chloroplast haplotypes found in Australia was identified in South African material only from plants collected in the Western Cape Province, near Cape Town. The other common chloroplast haplotype identified in Australia was also found in plants from this area, as well as other regions in South Africa. This suggested that the region around Cape Town may be the provenance of the invasive Australian lineage, though the possibility that L. ferocissimum was introduced to Australia from multiple localities could not be excluded.
A second set of genetic analyses using the more powerful next generation sequencing approach, genotyping-by-sequencing (GBS), was performed to better characterise the likely origins of Australian genotypes of L. ferocissimum (Gurdasani et al. in preparation). We compared 3409 SNPs across 442 L. ferocissimum samples from 64 localities across the entire distribution of the species in South Africa and Australia. Clear geographic genetic structuring was detected across South Africa, with distinct populations across the Eastern and Western Cape provinces. Our analyses indicated that the invasive L. ferocissimum plants in Australia originated from the Western Cape province, with plants from near Malmesbury (in the northern part of the species’ distribution) the closest genetic match to the Australian samples. Samples from Australia had similar levels of genetic diversity as those from South Africa, but there was no evidence of genetic structure across Australia. Our results suggested that the search for candidate biocontrol agents for L. ferocissimum should be focused in the Western Cape province.
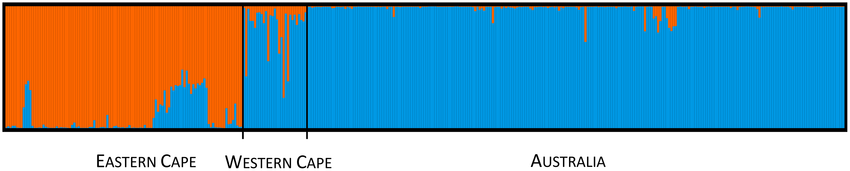
Australian Lycium ferocissimum plants are genomically most similar to plants from the Western Cape. STRUCTURE plot for L. ferocissimum samples collected from across its native distribution (South Africa) and introduced range (Australia). Each vertical bar represents an individual, with colour representing the inferred genomic cluster (McCulloch et al. 2023).
Undertertook bioclimatic models to identify optimal locations and conduct native range surveys and host-specificity tests for potential biocontrol agent(s) and import at least one potential agent in quarantine
Bioclimatic modelling
Bioclimatic models using the CLIMEX were developed (Kriticos et al. 2021). The simpler Match climates model was used to identify where to focus the search for candidate biocontrol agents in South Africa. It showed that L. ferocissimum is climatically well-matched to the Mediterranean and dry arid and semi-arid climate zones in South Africa, which accords with the rangelands in which it is an invasive problem in Australia.
Surprisingly there were little data on the climatic requirements and physiology of L. ferocissimum in the literature to inform the more sophisticated Compare Locations model. An experiment was thus conducted to measure the growth rate of L. ferocissimum at different temperatures to refine temperature growth-related parameters for the model. From this model, we extracted the Monthly Growth Index values in South Africa for guiding when and where to survey for natural enemies on L. ferocissimum. Similar values were extracted for Australia to guide when and where biocontrol agents should be released, so that L. ferocissimum is actively growing at the selected sites at the time of release, in order to increase chances of establishment.
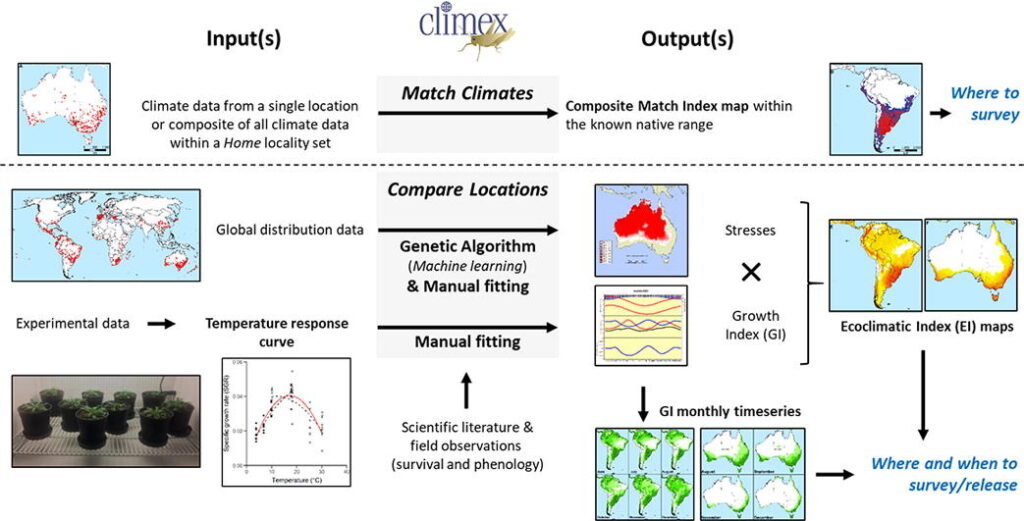
Framework for integrating CLIMEX ecoclimatic modelling into the exploration (native range surveys) and deployment (release strategies) stages of classical biological control programmes. The dotted line separates the simple Match Climates modelling approach from the more complicated but robust Compare Locations modelling approach (Kriticos et al. 2021).
Native range surveys
Pathogens
A comprehensive survey for diseases on L. ferocissimum was performed in October 2017 at 28 sites across the Eastern (13 sites) and Western (15 sites) Cape provinces of South Africa. Results from this survey are published in Ireland et al. (2019a). Disease symptoms caused by the rust fungus Puccinia rapipes were observed on L. ferocissimum at 4 of the 13 sites in the Eastern Cape and 10 of the 15 sites in the Western Cape. The rust fungus was not observed on any other Lycium species. The most severe rust symptoms were observed on L. ferocissimum at coastal sites in the Western Cape. Disease symptoms of any other primary pathogens were not observed at any of the sites surveyed.

The rust fungus Puccinia rapipes on African boxthorn in South Africa (photo courtesy of Alan Wood PPRI-ARC).
Two sites in each of the Western and Eastern Cape provinces were also visited multiple times between November 2016 and October 2017 to source material of P. rapipes to establish cultures in the Australian containment facility and track life cycle development. On each field visit, rust life cycle stages and evidence of any other disease symptoms were recorded. Uredinia, telia, spermogonia and aecia were observed on L. ferocissimum at both sites where the Eastern and Western Cape during the course of these visits, confirming that the rust fungus is a macrocyclic and autoicous (no alternate host) rust fungus (Ireland et al. 2019a).
Insects
Native range surveys for potential insect biocontrol agents on L. ferocissimum were performed over a two-year period in the Eastern Cape and Western Cape provinces of South Africa. In total, 96 insect species comprising 1315 individuals were collected from both provinces (Chari et al. 2020). Of the 96 species, three species, the leaf-chewing beetles Cassida distinguenda Spaeth (Chrysomelidae) and Cleta eckloni Mulsant (Coccinellidae) and the leaf-mining weevil Neoplatygaster serietuberculata Gyllenhal (Curculionidae) were prioritised as potential biocontrol agents based on their distribution, abundance, preliminary biology studies, pilot in-field host-specificity studies (in South Africa) and feeding preference.
Importation of candidate agents into quarantine
Pathogens
An accession of P. rapipes from the Western Cape Province of South Africa was imported in quarantine in Australia early 2017, but a culture could not be established because teliospores were dormant. Later in 2017 two additional shipments of rust-infected material were imported; an accession from the Eastern Cape Province in August and another accession from the Western Cape Province in October. Material from both accessions contained urediniospores that readily germinated, and a culture of each accession was established in quarantine. These two accessions were purified to generate single-uredinum isolates, bulked-up and used in a series of experiments.
Insects
Accessions of C. eckloni from the Eastern Cape and Western Cape provinces were imported into Australian quarantine in May 2019 and February 2020, respectively.
Host-specificity tests for potential biocontrol agents
The proposed list of non-target species for host-specificity testing of candidate biocontrol agents for L. ferocissimum was submitted to DAWE in December 2018 for posting on their website for feedback (Ireland et al. 2018).
Pathogens
A preliminary host-specificity study using two purified isolates of P. rapipes, from the Eastern and Western Cape provinces of South Africa, was performed in quarantine in Australia (results published in Ireland et al. 2019a). The experiments comprised two different chloroplast haplotypes of L. ferocissimum identified in Australia (McCulloch et al. 2020) and seven species closely related to the weed that occur in Australia. The L. ferocissimum haplotypes and the three Lycium species of Eurasian origin tested ‒ L. barbarum, L. chinense and L. ruthenicum ‒ were found to be susceptible to both isolates of P. rapipes used, while the Australian native L. australe was resistant. The three more distantly related species to L. ferocissimum tested were immune to the fungus: Hyoscyamus albus, Hyoscyamus aureus and Solanum aviculare. The susceptibility of Goji berry (L. barbarum) to P. rapipes was further confirmed by our collaborators in South Africa in a study performed under natural conditions.

A leaf of African boxthorn infected by the rust fungus Puccinia rapipes.
To better appreciate the implications of such results, further testing was suspended, and we undertook extensive stakeholder consultation. We first reviewed the known and potential economic and social importance of non-native Lycium species propagated and sold within Australia, ascertained by review of the literature, online searches and discussions with stakeholders. We then further consulted growers and retailers of Goji berry to identify possible concerns they would have if a weed biocontrol agent was to be released that could infect their Goji berry plants. With this background information provided, we asked the national Plant Health Committee (PHC) for advice on whether it would be acceptable for an exotic weed biocontrol agent targeting African boxthorn in Australia to also infect Goji berry. This committee is responsible for reviewing applications for release of weed biocontrol agents that are submitted via DAWE and thus is in the best position to provide initial advice on this issue. This consultation process indicated that Goji berry is an extremely low value and volume plant in Australia, and overall growers, wholesalers and retailers were not particularly concerned about the possible release of a biocontrol agent for the L. ferocissimum that would also affect Goji berry. Based on the responses received, including those from PHC, it was decided to resume work with P. rapipes.
Comprehensive host-specificity testing in quarantine began in September 2019 using one of the purified isolates (ex. Western Cape). The test list comprises a total of 31 closely related, non-target species in the family Solanaceae that occur in Australia (ornamental, weed and native). Most species are tested in at least two separate experiments using different accessions of each plant species, with L. ferocissimum plants used as positive controls in all experiments. Testing is on-going and thus far, 11 experiments have been conducted comprising 17 different species (Ireland et al. in preparation). Similarly, to results of the preliminary study, all the Lycium species non-native to Australia included in these experiments to date, including the target weed L. ferocissimum and the Eurasian Goji berries L. barbarum and L. ruthenicum were found to be susceptible to P. rapipes. In contrast, all accessions of the Australian native Lycium australe tested were found to be immune to the fungus, while all other species tested were either immune or displayed various levels of resistance to P. rapipes.
The host-specificity testing of the African boxthorn rust fungus, Puccinia rapipes, and the release application were completed during the reporting period. The release application was submitted to the Department of Agriculture, Water and the Environment on 27 November 2020.
Insects
Pilot in-field host specificity tests with the three promising candidate agents (C. eckloni, N. serietuberculata, C. distinguenda) were conducted on selected test plants at three sites in the Eastern Cape Province in South Africa: L. oxycarpum, L. barbarum, Solanum melongena (eggplant). There was generally very minimal or no spill over of the insects on the non-target plants. At only one of the sites, three C. eckloni and one C. distinguenda individuals were observed resting on L. oxycarpum and S. melongena, but without any signs of feeding.Preliminary host-specificity testing with C. eckloni and C. distinguenda was also conducted in a glasshouse at Rhodes University in South Africa. These tests showed that the insects have a host range restricted to the genus Lycium. Adult feeding and oviposition were recorded on L. barbarum, and both insects were able to complete their life cycle on this species.
Among the three prioritized agents, C. eckloni from a population in the Eastern Cape province was first imported into an Australian quarantine facility in February 2019 (and again in April and Mary 2019, to bolster colonies) (Fig. 8). Comprehensive no-choice host-specificity tests with this leaf-feeding beetle revealed that it feeds and reproduces on the three Lycium ‘goji berry’ species (L. chinense, L. barbarum and L. ruthenicum) as well as the native L. australe to the same extent observed on L. ferocissimum (Fig. 9).

Cleta eckloni. (A) adult, (B) eggs which are laid in clusters on the abaxial side of the leaves, (C) 1st instar larvae on top of eggs, (D) larvae with diagnostic branched processes on top of the damaged leaf with the distinct feeding pattern on the leaf, and (E-F) larvae of the last instar stage and newly pupated adult from the last instar stage (photo courtesy of Evans Mauda, Rhodes University).
As part of the investigations on C. eckloni we noticed morphological and molecular variation in this taxon in key diagnostic features between populations from the Eastern Cape and Western Cape provinces. This suggested that this taxon was perhaps a complex of cryptic species. In order to explicitly test this, we have commenced molecular characterisation of this species and, in Feb 2020, we imported a population of this species from the Western Cape to repeat the host-specificity testing undertaken with the Eastern Cape population. This testing will be part of the new Rural R&D for Profit project (Agrifutures Australia Project number: PRJ-12377). If the test results are similar to those with the Eastern Cape population, further testing with this insect will be discontinued and we will shift our focus to other candidate insect agents identified during native range surveys.
Explored options for integration of biocontrol with other management techniques
Long term effective control of L. ferocissimum requires a combination of treatments over many years due to the capacity of the species to regenerate from rootstock, stems and seed. Lycium ferocissimum seed is dispersed predominantly by birds and other fauna, and potential for re-infestation of sites from outside sources should be considered in management planning.
Physical control techniques
Physical control of L. ferocissimum includes winching, pulling, bulldozing, stick raking, blade ploughing and cultivation (Noble and Rose, 2013). These techniques are best used when L. ferocissimum plants are not carrying seed (or are carrying minimal seed). Otherwise, fresh seed is likely to be deposited into freshly disturbed soil. Winching and pulling are the lowest impact physical control techniques for situations where disturbance is a concern, such as where L. ferocissimum is growing within native vegetation. Bulldozing, stick raking and blade ploughing are suitable in less sensitive landscapes (e.g. pasture), and provide a rapid control method for moderate to heavy infestations.
Successful management of L. ferocissimum using the above techniques is dependent on follow-up application of herbicide. This includes cut-stump technique with immediate application of herbicide for any remaining base/roots after winching and pulling. For all physical control techniques, there is a need to return periodically and carry out foliar spray application and/or machine-based cut stump treatments until there is no regrowth or seedling presence (Noble and Rose, 2013).
Chemical control techniques
Chemical control of L. ferocissimum uses techniques including foliar spraying, cut-stump application (including mechanical cut-stump), stem injection, stem scrap or frilling (e.g. using chisel cuts), basal bark application, and soil-root zone application. Glyphosate, triclopyr, picloram, aminopyralid, hexazinone and tebuthiuron have all been trialed in the chemical control of L. ferocissimum. Appropriate formulations, mixes and applications of these chemicals are details in Noble and Rose (2013) and Noble et al. (2021).
Biocontrol
Biocontrol is likely to be an important component of landscape scale management of this species. The rust pathogen is likely to establish and perform optimally in wetter or more humid parts of the L. ferocissimum distribution in Australia, and in wetter years. Sites that fit these criteria might make good nursery sites, where the agent could initially establish and from where natural or assisted dispersal of the agent could occur.
Integration of management tactics
At the landscape scale, minimising the disruptions of biocontrol agents by other control tactics (e.g. avoiding spraying plants in nursery sites with herbicides) will require coordination among land managers recommending/deploying management tactics for L. ferocissimum. In addition, there are likely to be many circumstances where the pathogen (and other biocontrol agents) may act in concert with physical and chemical management. For example, it could be of value to trial the use of the pathogen as a follow-up treatment to control recruiting seedlings in place of herbicide applications. Similarly, the timing of releases of the pathogen with periods of regrowth of the weed following physical treatment, may also aid the integration of biocontrol to be another chronic stressor for this weed.
References
Chari LD, Mauda EV, Martin GD, Raghu S (2020) Insect herbivores associated with Lycium ferocissimum (Solanaceae) in South Africa and their potential as biological control agents in Australia. African Entomology 28(2): 359–73. https://doi.org/10.4001/003.028.0359
GBIF.org (24th July 2018) GBIF Occurrence Download https://doi.org/10.15468/dl.pbd67p.
Ireland KB, Hunter GC, Wood A, Delaisse C, Morin L (2019a) Evaluation of the rust fungus Puccinia rapipes for biological control of Lycium ferocissimum (African boxthorn) in Australia: life cycle, taxonomy and pathogenicity. Fungal Biol. 123: 811–23. https://doi.org/10.1016/j.funbio.2019.08.007
Ireland KB, Rafter M, Kumaran N, Raghu S, Morin L (2019b) Stakeholder survey reveals priorities for African boxthorn biocontrol research in Australia. Biocontrol Sci. Technol. 29:1123–28. https://doi.org/10.1080/09583157.2019.1656166
Ireland KB, Rafter MA, Raghu S, Morin L (2018) Proposed plant host test list for assessing risk of candidate biological control agents for Lycium ferocissimum. Prepared by CSIRO.
Kriticos DL, Ireland KB, Morin L, Kumaran N, Rafter MA, Ota N, Raghu S (2021) Integrating ecoclimatic niche modelling methods into classical biological control programmes. Biological Control, 160, 104667. https://doi.org/10.1016/j.biocontrol.2021.104667
Levin RA, Shak JR, Miller JS, Bernardello G, Venter AM Evolutionary relationships in tribe Lycieae (Solanaceae). In, 2007. International Society for Horticultural Science (ISHS), Leuven, Belgium, pp 225-240. https://doi:10.17660/ActaHortic.2007.745.9
McCulloch GA, Mauda EV, Chari LD, Martin GD, Gurdasani K, Morin L, Walter GH, Raghu S (2020) Genetic diversity and morphological variation in African boxthorn (Lycium ferocissimum) – Characterising the target weed for biological control. Biological Control 143:104206. https://doi.org/10.1016/j.biocontrol.2020.104206
McCulloch GA, Gurdasani K, Hereward JP, Morin L, Walter GH, Raghu S (2023). Invasion history of Lycium ferocissimum in Australia: The impact of admixture on genetic diversity and differentiation. Diversity and Distributions, 29: 879–91. https://doi.org/10.1111/ddi.13702
Noble MR, Rose M (2013) African Boxthorn National Best Practice Manual: Managing African boxthorn (Lycium ferocissimum) in Australia. Davenport, Tasmania: Tasmanian Department of Primary Industries, Parks, Water and Environment
Noble M, Adair R, Ireland K (2021). Biology of Invasive Plants 2. Lycium ferocissimum Miers. Invasive Plant Science and Management, 14(2): 41-56. https://doi:10.1017/inp.2021.13
Venter AM (2000) Taxonomy of the genus Lycium L. (Solanaceae) in Africa. University of the Orange Free State.
